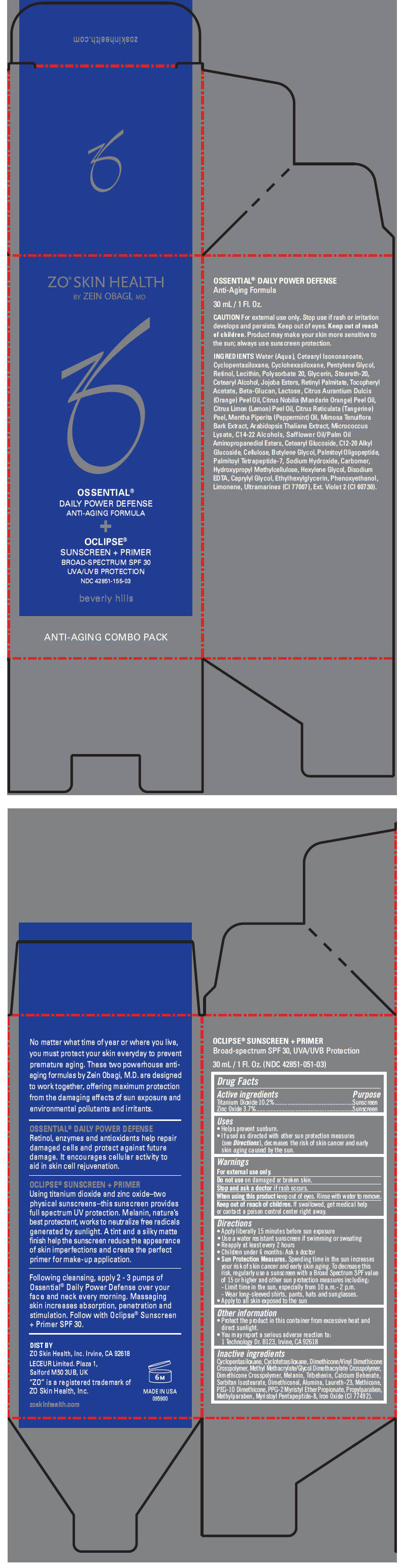
| NDC | 42851-051-03, 42851-155-03 |
| Set ID | 2d99bde7-2ecb-4609-80c4-739f08b9d1eb |
| Category | HUMAN OTC DRUG LABEL |
| Packager | ZO Skin Health, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- -
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- -
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Apply to all skin exposed to the sun
- Other information
-
Inactive ingredients
Cyclopentasiloxane, Cyclotetrasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Dimethicone Crosspolymer, Melanin, Tribehenin, Calcium Behenate, Sorbitan Isostearate, Dimethiconol, Alumina, Laureth-23, Methicone, PEG-10 Dimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Methylparaben, Myristoyl Pentapeptide-8, Iron Oxide (CI 77492).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA AND OCLIPSE SUNSCREEN PLUS PRIMER BROAD SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-155-03 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PUMP 30 mL Part 2 1 BOTTLE, PUMP 30 mL Part 1 of 2 OCLIPSE SUNSCREEN PLUS PRIMER BROAD-SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide and zinc oxide lotionProduct Information Item Code (Source) NDC:42851-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) TITANIUM DIOXIDE 102 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) ZINC OXIDE 37 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 4 (UNII: CZ227117JE) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) TRIBEHENIN (UNII: 8OC9U7TQZ0) CALCIUM BEHENATE (UNII: J5VFA9V6YG) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) ALUMINUM OXIDE (UNII: LMI26O6933) LAURETH-23 (UNII: N72LMW566G) METHICONE (20 CST) (UNII: 6777U11MKT) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-051-03 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/01/2012 Part 2 of 2 OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CETEARYL ISONONANOATE (UNII: P5O01U99NI) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR RETINOL (UNII: G2SH0XKK91) INGR EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR STEARETH-20 (UNII: L0Q8IK9E08) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR JOJOBA BUTTER (UNII: XIA46H803R) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR CURDLAN (UNII: 6930DL209R) INGR LACTOSE (UNII: J2B2A4N98G) INGR ORANGE OIL (UNII: AKN3KSD11B) INGR MANDARIN OIL (UNII: NJO720F72R) INGR LEMON OIL (UNII: I9GRO824LL) INGR PEPPERMINT OIL (UNII: AV092KU4JH) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) INGR C14-22 ALCOHOLS (UNII: B1K89384RJ) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/01/2012 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Owen Biosciences 790003045 MANUFACTURE(42851-155) Establishment Name Address ID/FEI Business Operations PakLab, Inc. 177711082 MANUFACTURE(42851-155)