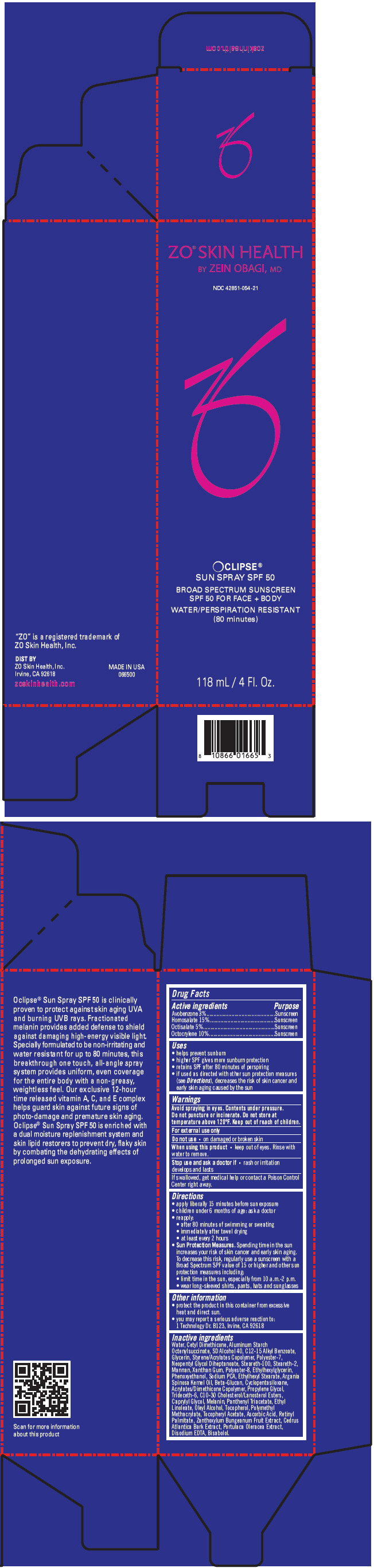
| NDC | 42851-054-21 |
| Set ID | c86ee907-d7b1-497d-b3a1-47484bf118b3 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | ZO Skin Health, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- retains SPF after 80 minutes of perspiring
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- children under 6 months of age: ask a doctor
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Water, Cetyl Dimethicone, Aluminum Starch Octenylsuccinate, SD Alcohol 40, C12-15 Alkyl Benzoate, Glycerin, Styrene/Acrylates Copolymer, Polyester-7, Neopentyl Glycol Diheptanoate, Steareth-100, Steareth-2, Mannan, Xanthan Gum, Polyester-8, Ethylhexylglycerin, Phenoxyethanol, Sodium PCA, Ethylhexyl Stearate, Argania Spinosa Kernel Oil, Beta-Glucan, Cyclopentasiloxane, Acrylates/Dimethicone Copolymer, Propylene Glycol, Trideceth-6, C10-30 Cholesterol/Lanosterol Esters, Caprylyl Glycol, Melanin, Panthenyl Triacetate, Ethyl Linoleate, Oleyl Alcohol, Tocopherol, Polymethyl Methacrylate, Tocopheryl Acetate, Ascorbic Acid, Retinyl Palmitate, Zanthoxylum Bungeanum Fruit Extract, Cedrus Atlantica Bark Extract, Portulaca Oleracea Extract, Disodium EDTA, Bisabolol.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH OCLIPSE SUN SPF 50 BROAD SPECTRUM SUNSCREEN SPF 50 FOR FACE PLUS BODY WATER/PERSPIRATION RESISTANT (80 MINUTES)
avobenzone, homosalate, octisalate, and octocrylene aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-054 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 g in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 150 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 g in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETYL DIMETHICONE 45 (UNII: IK315POC44) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALCOHOL (UNII: 3K9958V90M) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) POLYESTER-7 (UNII: 0841698D2F) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) STEARETH-100 (UNII: 4OH5W9UM87) STEARETH-2 (UNII: V56DFE46J5) YEAST MANNAN (UNII: 91R887N59P) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) ARGAN OIL (UNII: 4V59G5UW9X) CURDLAN (UNII: 6930DL209R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRIDECETH-6 (UNII: 3T5PCR2H0C) C10-30 CHOLESTEROL/LANOSTEROL ESTERS (UNII: 137SL7IL0Y) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MELAMINE (UNII: N3GP2YSD88) PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) ETHYL LINOLEATE (UNII: MJ2YTT4J8M) OLEYL ALCOHOL (UNII: 172F2WN8DV) TOCOPHEROL (UNII: R0ZB2556P8) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBIC ACID (UNII: PQ6CK8PD0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ZANTHOXYLUM BUNGEANUM FRUIT (UNII: 3CIP16A418) CEDRUS ATLANTICA BARK (UNII: ITP1Q41UPF) PURSLANE (UNII: M6S840WXG5) EDETATE DISODIUM (UNII: 7FLD91C86K) LEVOMENOL (UNII: 24WE03BX2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-054-21 1 in 1 CARTON 05/01/2015 1 118 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/01/2015 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Product Quest 927768135 MANUFACTURE(42851-054)