| NDC | 13811-582-30 |
| Set ID | 4f7c0fa9-dcc5-42d7-b2f1-95e2e2aa94f3 |
| Category | DIETARY SUPPLEMENT |
| Packager | Trigen Laboratories, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
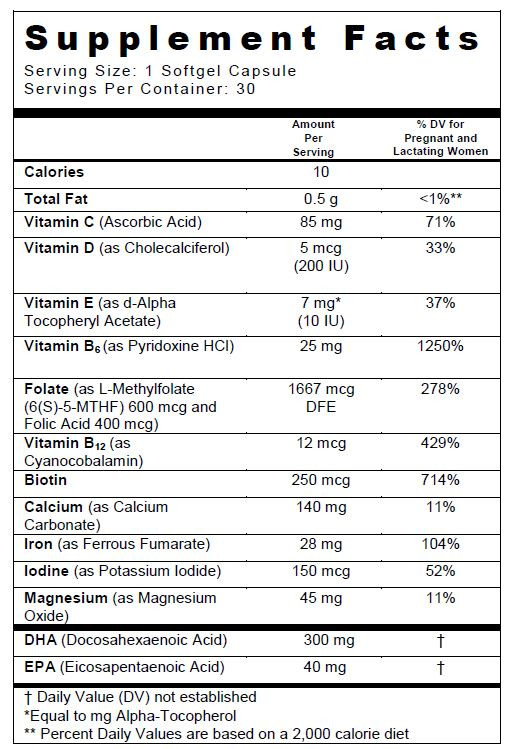
SUPPLEMENT FACTS

Other Ingredients: Orange Cream Flavor O.S., Citric Acid, Biomin Potassium Citrate, Soy Lecithin, Yellow Beeswax
Shell Ingredients: Bovine Gelatin, Glycerin, Purified Water, Sorbitol, Titanium Dioxide, Ethyl Vanillin, FD&C Yellow #5, FD&C Blue #1, and Caramel.
THIS PRODUCT CONTAINS SOY AND FISH OIL (ANCHOVY).Zatean™-Pn Plus is a prescription prenatal/postnatal multivitamin/ mineral softgel with an essential fatty acid and is indicated for the supplemental requirements of patients with nutritional deficiencies or are in need of nutritional supplementation.
- CONTRAINDICATIONS
-
WARNING
Administration of omega-3 fatty acids – including DHA, should be avoided in patients with inherited or acquired bleeding diatheses, including those taking anticoagulants.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. -
PRECAUTIONS
General: Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. Reduced folates may be less likely than folic acid to mask vitamin B12 deficiency. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible patients. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
-
DRUG INTERACTIONS
Pyridoxine hydrochloride should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid, as well as possibly the use of other forms of folates, including reduced folates. Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with cyanocobalamin.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-877-482-3788 or FDA at 1-800-FDA-1088.
Rev. 05/2020
Customer Service: 1-877-482-3788
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807
www.trigenlab.com
- PRINCIPAL DISPLAY PANEL - 30 Softgel Bottle Label
-
INGREDIENTS AND APPEARANCE
ZATEAN-PN PLUS
levomefolate calcium, folic acid, ascorbic acid, cholecalciferol, .alpha.-tocopherol, pyridoxine, cyanocobalamin, biotin, calcium carbonate, ferrous fumarate, potassium iodide, magnesium oxide, doconexent, and icosapent capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-582 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMEFOLIC ACID (UNII: 8S95DH25XC) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 600 ug folic acid (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) folic acid 400 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 85 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 200 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 10 [iU] PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug Biotin (UNII: 6SO6U10H04) (Biotin - UNII:6SO6U10H04) Biotin 250 ug calcium carbonate (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) calcium carbonate 140 mg ferrous fumarate (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 28 mg potassium iodide (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) potassium iodide 150 ug magnesium oxide (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) magnesium oxide 45 mg Doconexent (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) Doconexent 300 mg Icosapent (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) Icosapent 40 mg Inactive Ingredients Ingredient Name Strength caramel (UNII: T9D99G2B1R) ethyl vanillin (UNII: YC9ST449YJ) FD&C Blue no. 1 (UNII: H3R47K3TBD) FD&C Yellow no. 5 (UNII: I753WB2F1M) gelatin (UNII: 2G86QN327L) glycerin (UNII: PDC6A3C0OX) lecithin, soybean (UNII: 1DI56QDM62) water (UNII: 059QF0KO0R) sorbitol (UNII: 506T60A25R) titanium dioxide (UNII: 15FIX9V2JP) potassium citrate anhydrous (UNII: 86R1NVR0HW) yellow wax (UNII: 2ZA36H0S2V) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ORANGE OIL (UNII: AKN3KSD11B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-582-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 05/01/2010 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 26 mm Labeler - Trigen Laboratories, LLC (830479668)