| NDC | 23319-0013-1, 23319-0014-1, 23319-0015-1, 23319-0016-1 |
| Set ID | 785f2b2e-a523-6278-e053-2991aa0a8f07 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Yanbal USA, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
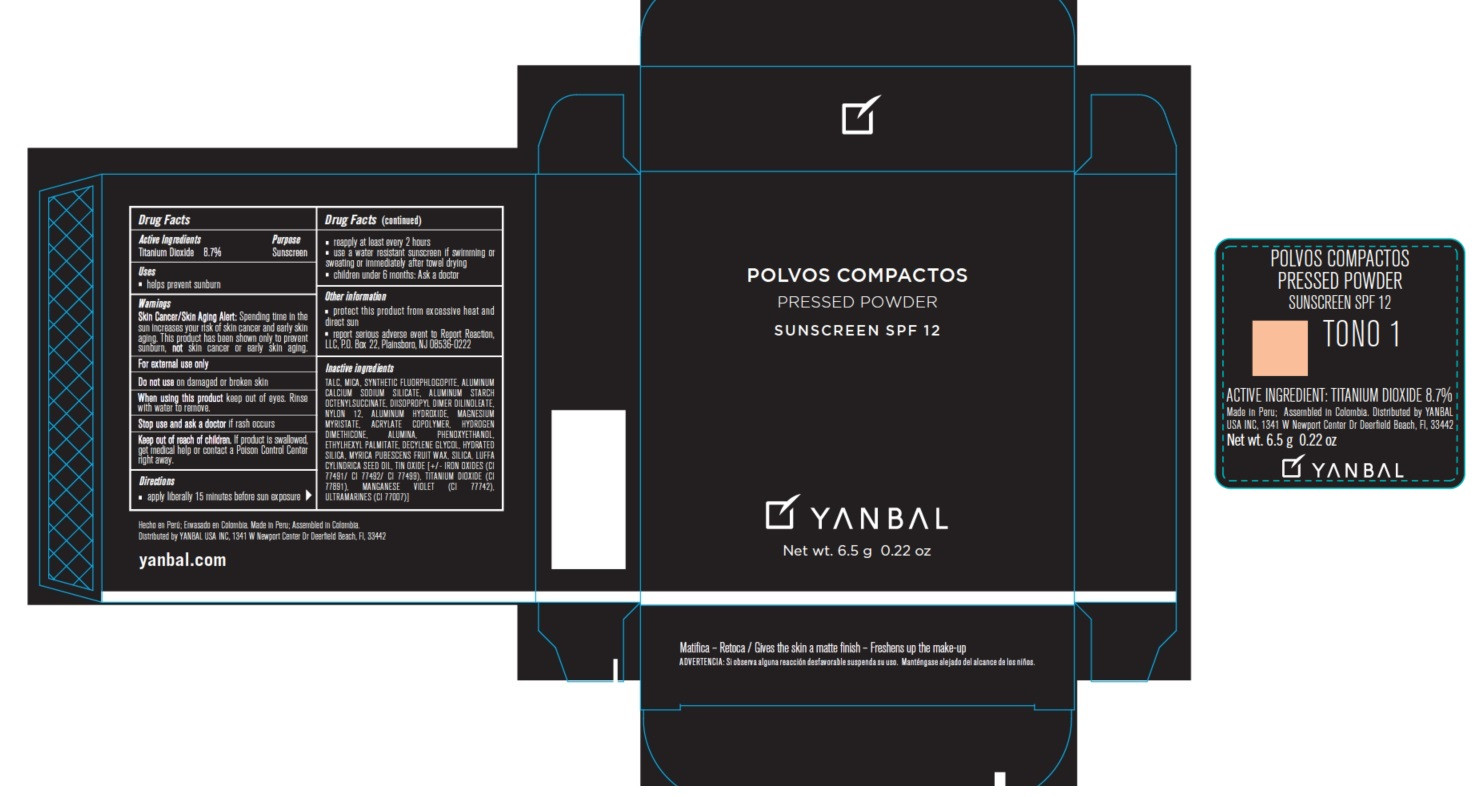
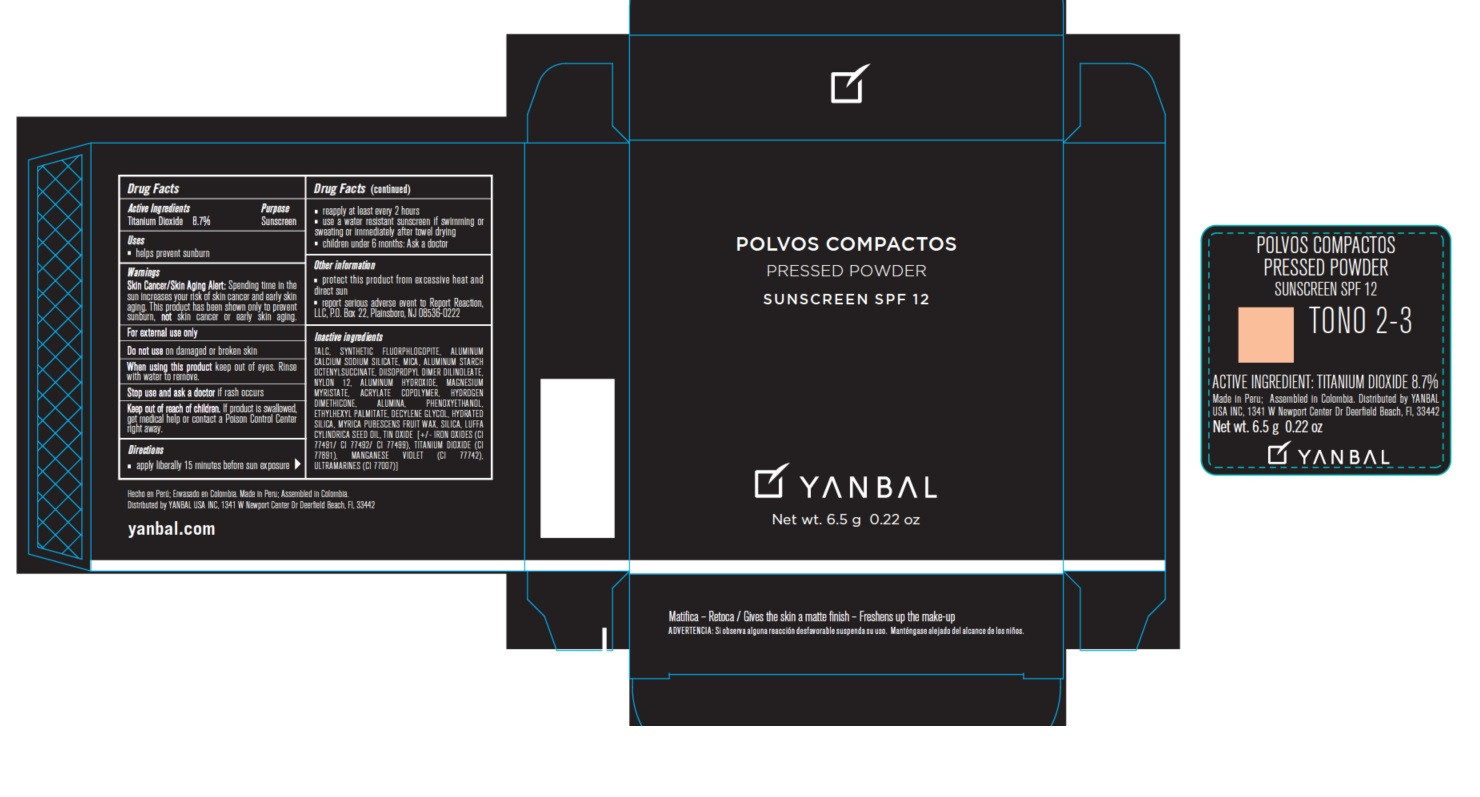
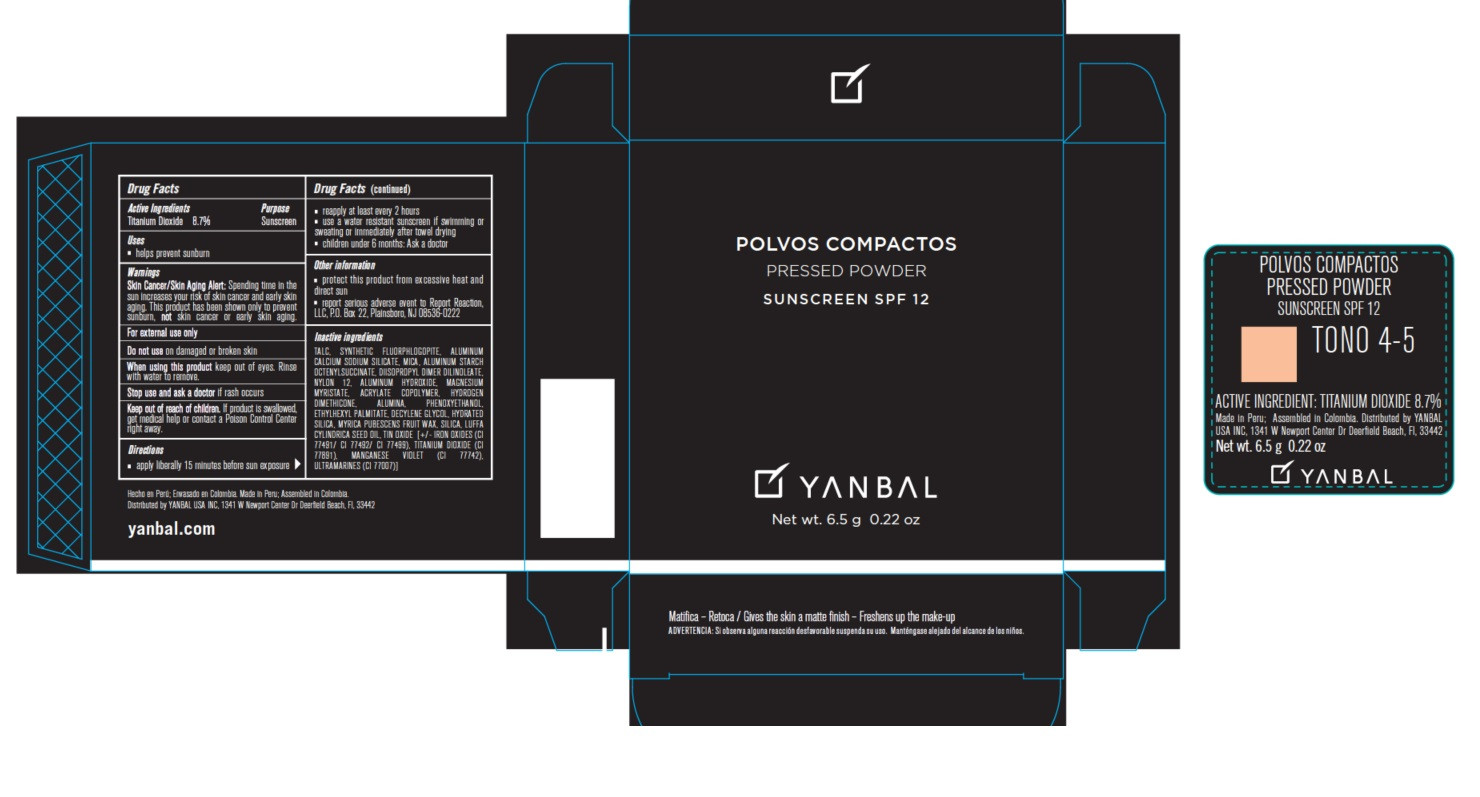
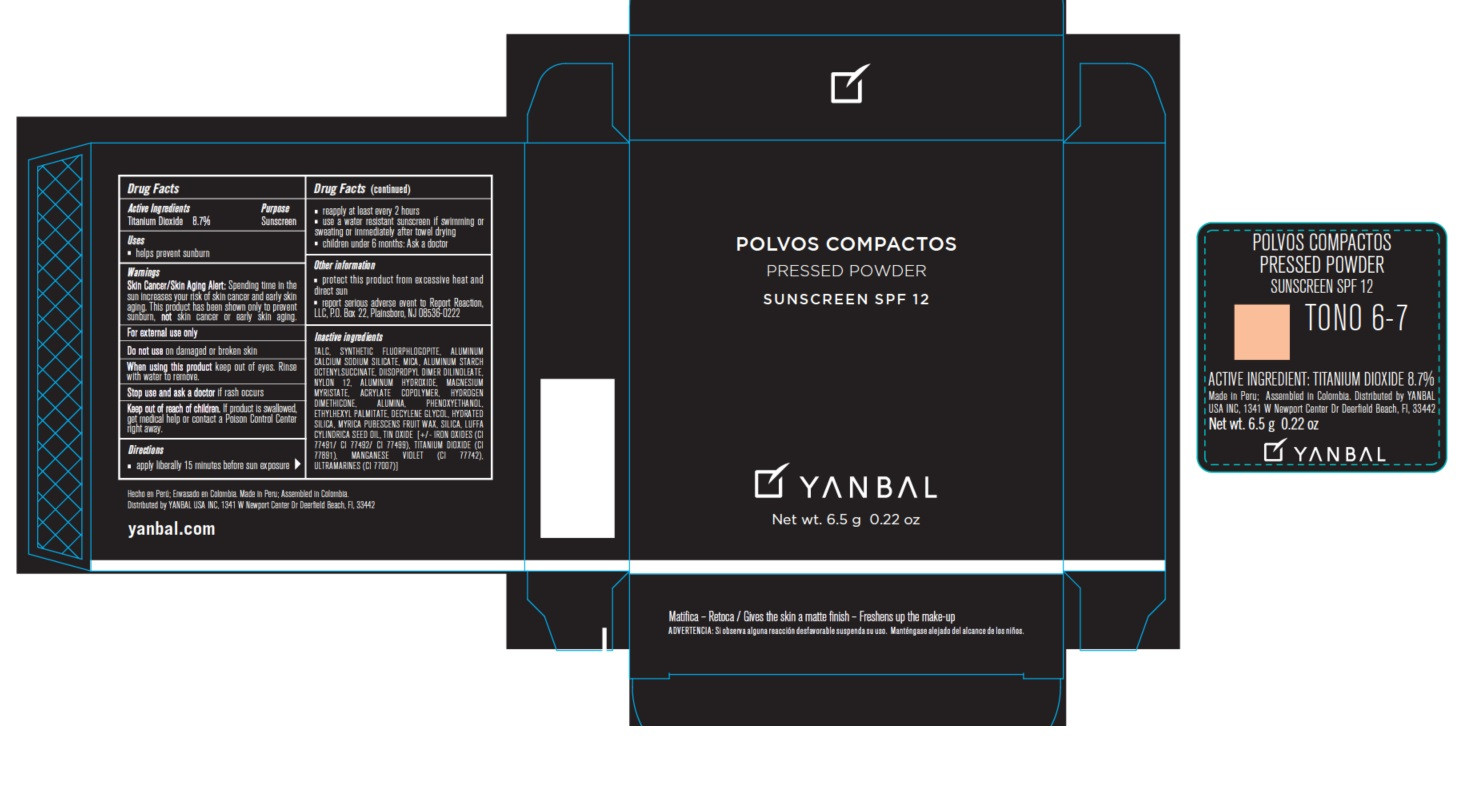
- Active ingredients
- Uses
- Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of the reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Close - Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating or immediately after towel drying
- children under 6 months: Ask a doctor.
- Other information
- protect this product from excessive heat and direct sun
- report serious adverse event to Report Reaction, LLC, P.O. Box 22, Plainsboro, NJ 08536-0222
- Inactive ingredients
TALC, MICA, SYNTHETIC FLUORPHLOGOPITE, ALUMINUM CALCIUM SODIUM SILICATE, ALUMINUM STARCH OCTENYLSUCCINATE, DIISOPROPYL DIMER DILINOLEATE, NYLON 12, ALUMINUM HYDROXIDE, MAGNESIUM MYRISTATE, ACRYLATE COPOLYMER, HYDROGEN DIMETHICONE, ALUMINA, PHENOXYETHANOL, ETHYLHEXYL PALMITATE, DECYLENE GLYCOL, HYDRATED SILICA, MYRICA PUBESCENS FRUIT WAX, SILICA, LUFFA CYLINDRICA SEED OIL, TIN OXIDE, IRON OXIDES (CI 77491/CI 77492/ CI 77499), TITANIUM DIOXIDE (CI 77891) MANGANESE VIOLET (CI 77742), ULTRAMARINES (CI 77007).
Close - Company information
- Product Packaging - Tono 1
- Product Packaging - Tono 2-3
- Procut Packaging - Tono 4-5
- Product Packaging - Tono 6-7
- INGREDIENTS AND APPEARANCE
YANBAL POLVOS COMPACTOS PRESSED SUNSCREEN SPF 12 TONO 4-5
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23319-0015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 87 mg in 1 g Inactive Ingredients Ingredient Name Strength LUFFA AEGYPTIACA SEED OIL (UNII: 1A281RJ859) STANNIC OXIDE (UNII: KM7N50LOS6) DECYLENE GLYCOL (UNII: S57M60MI88) ULTRAMARINE BLUE (UNII: I39WR998BI) MAGNESIUM MYRISTATE (UNII: Z1917F0578) FERROUS OXIDE (UNII: G7036X8B5H) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDRATED SILICA (UNII: Y6O7T4G8P9) MORELLA PUBESCENS FRUIT WAX (UNII: 2N43GS24LV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) MICA (UNII: V8A1AW0880) ALUMINUM OXIDE (UNII: LMI26O6933) TALC (UNII: 7SEV7J4R1U) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) NYLON-12 (UNII: 446U8J075B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23319-0015-1 1 in 1 CARTON 11/15/2018 1 6.5 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/15/2018 YANBAL POLVOS COMPACTOS PRESSED SUNSCREEN SPF 12 TONO 6-7
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23319-0016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 87 mg in 1 g Inactive Ingredients Ingredient Name Strength LUFFA AEGYPTIACA SEED OIL (UNII: 1A281RJ859) STANNIC OXIDE (UNII: KM7N50LOS6) DECYLENE GLYCOL (UNII: S57M60MI88) ULTRAMARINE BLUE (UNII: I39WR998BI) MAGNESIUM MYRISTATE (UNII: Z1917F0578) FERROUS OXIDE (UNII: G7036X8B5H) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDRATED SILICA (UNII: Y6O7T4G8P9) MORELLA PUBESCENS FRUIT WAX (UNII: 2N43GS24LV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) NYLON-12 (UNII: 446U8J075B) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) MICA (UNII: V8A1AW0880) ALUMINUM OXIDE (UNII: LMI26O6933) TALC (UNII: 7SEV7J4R1U) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23319-0016-1 1 in 1 CARTON 11/15/2018 1 6.5 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/15/2018 YANBAL POLVOS COMPACTOS PRESSED SUNSCREEN SPF 12 TONO 1
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23319-0013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 87 mg in 1 g Inactive Ingredients Ingredient Name Strength LUFFA AEGYPTIACA SEED OIL (UNII: 1A281RJ859) STANNIC OXIDE (UNII: KM7N50LOS6) DECYLENE GLYCOL (UNII: S57M60MI88) ULTRAMARINE BLUE (UNII: I39WR998BI) MAGNESIUM MYRISTATE (UNII: Z1917F0578) FERROUS OXIDE (UNII: G7036X8B5H) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDRATED SILICA (UNII: Y6O7T4G8P9) MORELLA PUBESCENS FRUIT WAX (UNII: 2N43GS24LV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) NYLON-12 (UNII: 446U8J075B) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) MICA (UNII: V8A1AW0880) ALUMINUM OXIDE (UNII: LMI26O6933) TALC (UNII: 7SEV7J4R1U) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23319-0013-1 1 in 1 CARTON 11/15/2018 1 6.5 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/15/2018 YANBAL POLVOS COMPACTOS PRESSED SUNSCREEN SPF 12 TONO 2-3
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23319-0014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 87 mg in 1 g Inactive Ingredients Ingredient Name Strength MANGANESE VIOLET (UNII: 72M48QQV8Q) LUFFA AEGYPTIACA SEED OIL (UNII: 1A281RJ859) STANNIC OXIDE (UNII: KM7N50LOS6) DECYLENE GLYCOL (UNII: S57M60MI88) MAGNESIUM MYRISTATE (UNII: Z1917F0578) FERROUS OXIDE (UNII: G7036X8B5H) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDRATED SILICA (UNII: Y6O7T4G8P9) MORELLA PUBESCENS FRUIT WAX (UNII: 2N43GS24LV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM OXIDE (UNII: LMI26O6933) NYLON-12 (UNII: 446U8J075B) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TALC (UNII: 7SEV7J4R1U) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23319-0014-1 1 in 1 CARTON 11/15/2018 1 6.5 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/15/2018 Labeler - Yanbal USA, Inc. (001801369) Establishment Name Address ID/FEI Business Operations Yanbal de Colombia S.A.S. 880215657 manufacture(23319-0013, 23319-0014, 23319-0015, 23319-0016) CloseEstablishment Name Address ID/FEI Business Operations Unique S.A. 934396326 manufacture(23319-0013, 23319-0014, 23319-0015, 23319-0016)