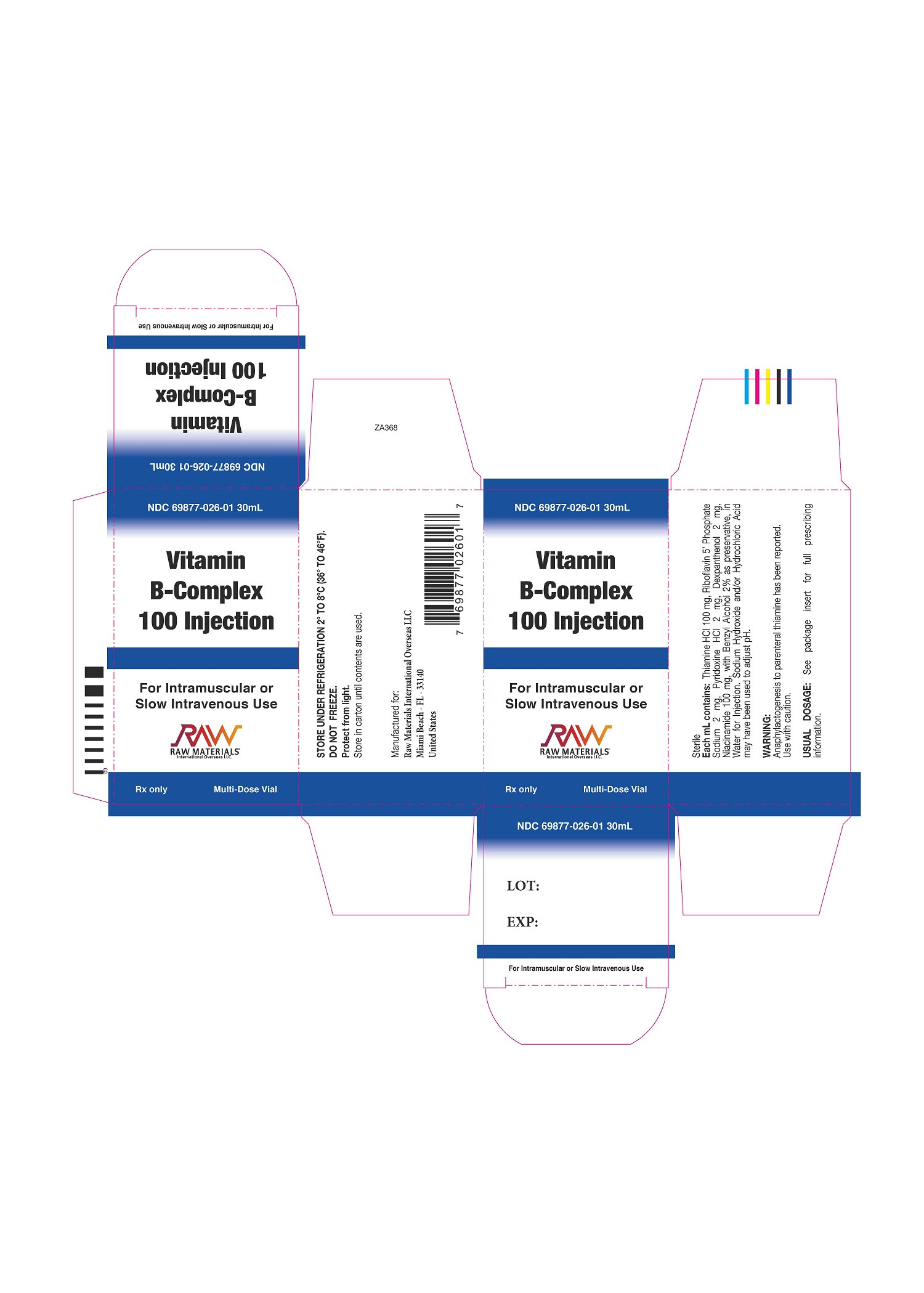
| NDC | 69877-026-01 |
| Set ID | 1a3a8ac6-39a6-6525-e054-00144ff8d46c |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Raw Materials International Overseas LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Vitamin B-Complex 100 Injection is a sterile solution for intramuscular or slow intravenous injection comprised of vitamins which may be categorized as belonging to the vitamin B complex group.
Each mL contains: Thiamine Hydrochloride 100 mg, Riboflavin 5’ Phosphate Sodium 2 mg, Pyridoxine Hydrochloride 2 mg, Dexpanthenol 2 mg, Niacinamide 100 mg, with Benzyl Alcohol 2% as preservative, in Water for Injection. Sodium Hydroxide and/or Hydrochloric Acid may have been used to adjust pH.
-
INDICATIONS & USAGE
In disorders requiring parenteral administration of vitamins, i.e. pre- and post-operative treatment, when requirements are increased as in fever, severe burns, increased metabolism, pregnancy, gastrointestinal disorders interfering with intake or absorption of vitamins, prolonged or wasting diseases, alcoholism and where other deficiencies exist.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
-
ADVERSE REACTIONS
Mild transient diarrhea, polycythemia vera, peripheral vascular thrombosis, itching transitory exanthema, feeling of swelling of entire body, anaphylactic shock and death. Sensitivity to the ingredients listed may occur (see WARNINGS). Use should be discontinued upon observance of any untoward reaction. Pain upon intramuscular injection may be noted.
-
DOSAGE & ADMINISTRATION
Usually 0.25 to 2 mL by intramuscular or slow intravenous injection. High concentrations given intravenously may be diluted using parenteral infusion solutions. (See PRECAUTIONS).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit (See HOW SUPPLIED).
-
HOW SUPPLIED
Vitamin B-Complex 100 Injection
Rx Only
NDC 69877-026-01 30 mL Multi-Dose Vial, individually boxed.
Phase separation due to reduced solubility can occur under certain conditions of shipping or storage (e.g. accidental freezing), which may produce visible particles. Do not use product if these do not redissolve on warming to body temperature and shaking well.
Refrigeration of the product may cause darkening of the solution due to the riboflavin content. The colour does not affect the safety or efficacy of the product.
PROTECT FROM LIGHT: Store in carton until contents are used. Store under refrigeration 2° to 8°C (36° to 46°F). Do not permit to freeze.
Manufactured for:
Raw Materials International Overseas LLC
Miami Beach, FL 33140United States
Rev. 07/15
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN B COMPLEX
thiamine hydrochloride, riboflavin 5 phosphate sodium, pyridoxine hydrochloride, dexpanthenol and niacinamide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69877-026 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 2 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 2 mg in 1 mL DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 2 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 100 mg in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 20 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69877-026-01 1 in 1 BOX 1 30 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/06/2015 Labeler - Raw Materials International Overseas LLC (079829477)