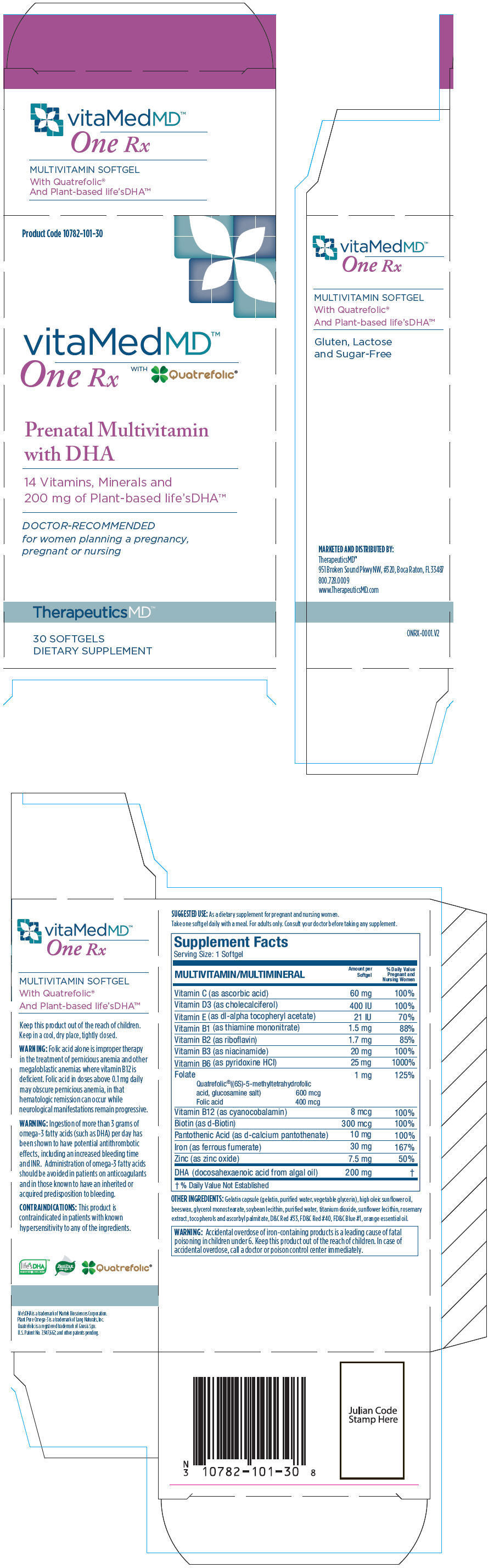
| NDC | 10782-101-30 |
| Set ID | c321dba6-eae6-42fa-8ff9-bdfa6ffabcd9 |
| Category | DIETARY SUPPLEMENT |
| Packager | vitaMedMD |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- DESCRIPTION
-
Supplement Facts
Serving Size: 1 Softgel
MULTIVITAMIN/MULTIMINERAL Amount per softgel % Daily Value Pregnant and Nursing Women - *
- % Daily Value Not Established
Vitamin C (as ascorbic acid) 60 mg 100% Vitamin D3 (as cholecalciferol) 400 IU 100% Vitamin E (as dl-alpha tocopheryl acetate) 21 IU 70% Vitamin B1 (as thiamine mononitrate) 1.5 mg 88% Vitamin B2 (as riboflavin) 1.7 mg 85% Vitamin B3 (as niacinamide) 20 mg 100% Vitamin B6 (as pyridoxine HCI) 25 mg 1000% Folate 1 mg 125% Quatrefolic®((6S)-5-methyltetrahydrofolic
acid, glucosamine salt) 600 mcg
Folic acid 400 mcgVitamin B12 (as cyanocobalamin) 8 mcg 100% Biotin (as d-Biotin) 300 mcg 100% Pantothenic Acid (as d-calcium pantothenate) 10 mg 100% Iron (as ferrous fumerate) 30 mg 167% Zinc (as zinc oxide) 7.5 mg 50% DHA (docosahexaenoic acid from algal oil) 200 mg * -
OTHER INGREDIENTS
Gelatin capsule (gelatin, purified water, vegetable glycerin), high oleic sunflower oil, beeswax, glycerol monostearate, soybean lecithin, purified water, titanium dioxide, sunflower lecithin, rosemary extract, tocopherols and ascorbyl palmitate, D&C Red #33, FD&C Red #40, FD&C Blue #1, orange essential oil.
-
INDICATIONS
vitaMedMD™ One Rx is a dietary supplement with DHA indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and non-lactating mothers. vitaMedMD™ One Rx can also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
- WARNINGS
-
WARNING
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
-
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SAFE HANDLING WARNING
- STATEMENT OF IDENTITY
- PRINCIPAL DISPLAY PANEL - 30 Softgel Bottle Carton
-
INGREDIENTS AND APPEARANCE
VITAMEDMD ONE RX
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, 5-methyltetrahydrofolic acid, folic acid, cyanocobalamin, biotin, calcium pantothenate, ferrous fumarate, zinc oxide, and doconexent capsule, gelatin coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:10782-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ascorbic acid (UNII: PQ6CK8PD0R) (ascorbic acid - UNII:PQ6CK8PD0R) ascorbic acid 60 mg cholecalciferol (UNII: 1C6V77QF41) (cholecalciferol - UNII:1C6V77QF41) cholecalciferol 400 [iU] .alpha.-tocopherol acetate, dl- (UNII: WR1WPI7EW8) (.alpha.-tocopherol, dl- - UNII:7QWA1RIO01) .alpha.-tocopherol acetate, dl- 21 [iU] thiamine mononitrate (UNII: 8K0I04919X) (thiamine ion - UNII:4ABT0J945J) thiamine mononitrate 1.5 mg riboflavin (UNII: TLM2976OFR) (riboflavin - UNII:TLM2976OFR) riboflavin 1.7 mg niacinamide (UNII: 25X51I8RD4) (niacinamide - UNII:25X51I8RD4) niacinamide 20 mg pyridoxine hydrochloride (UNII: 68Y4CF58BV) (pyridoxine - UNII:KV2JZ1BI6Z) pyridoxine hydrochloride 25 mg 5-METHYLTETRAHYDROFOLIC ACID (UNII: TYK22LML8F) (5-METHYLTETRAHYDROFOLIC ACID - UNII:TYK22LML8F) 5-METHYLTETRAHYDROFOLIC ACID 600 ug folic acid (UNII: 935E97BOY8) (folic acid - UNII:935E97BOY8) folic acid 400 ug cyanocobalamin (UNII: P6YC3EG204) (cyanocobalamin - UNII:P6YC3EG204) cyanocobalamin 8 ug biotin (UNII: 6SO6U10H04) (biotin - UNII:6SO6U10H04) biotin 300 ug calcium pantothenate (UNII: 568ET80C3D) (pantothenic acid - UNII:19F5HK2737) calcium pantothenate 10 mg ferrous fumarate (UNII: R5L488RY0Q) (ferrous cation - UNII:GW89581OWR) ferrous fumarate 30 mg zinc oxide (UNII: SOI2LOH54Z) (zinc oxide - UNII:SOI2LOH54Z) zinc oxide 7.5 mg doconexent (UNII: ZAD9OKH9JC) (doconexent - UNII:ZAD9OKH9JC) doconexent 200 mg Inactive Ingredients Ingredient Name Strength gelatin (UNII: 2G86QN327L) water (UNII: 059QF0KO0R) yellow wax (UNII: 2ZA36H0S2V) glyceryl monostearate (UNII: 230OU9XXE4) lecithin, soybean (UNII: 1DI56QDM62) titanium dioxide (UNII: 15FIX9V2JP) tocopherol (UNII: R0ZB2556P8) ascorbyl palmitate (UNII: QN83US2B0N) D&C red no. 33 (UNII: 9DBA0SBB0L) FD&C red no. 40 (UNII: WZB9127XOA) FD&C blue no. 1 (UNII: H3R47K3TBD) Glycerin (UNII: PDC6A3C0OX) Sunflower Oil (UNII: 3W1JG795YI) Rosemary (UNII: IJ67X351P9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:10782-101-30 30 in 1 BOX, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 04/15/2012 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 23 mm Labeler - vitaMedMD (019920270) Establishment Name Address ID/FEI Business Operations vitaMedMD 019920270 MANUFACTURE(10782-101)