| NDC | 65121-301-01 |
| Set ID | 33f32537-b148-48f3-a732-1f7c2f092f00 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Pure Source, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- DESCRIPTION
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
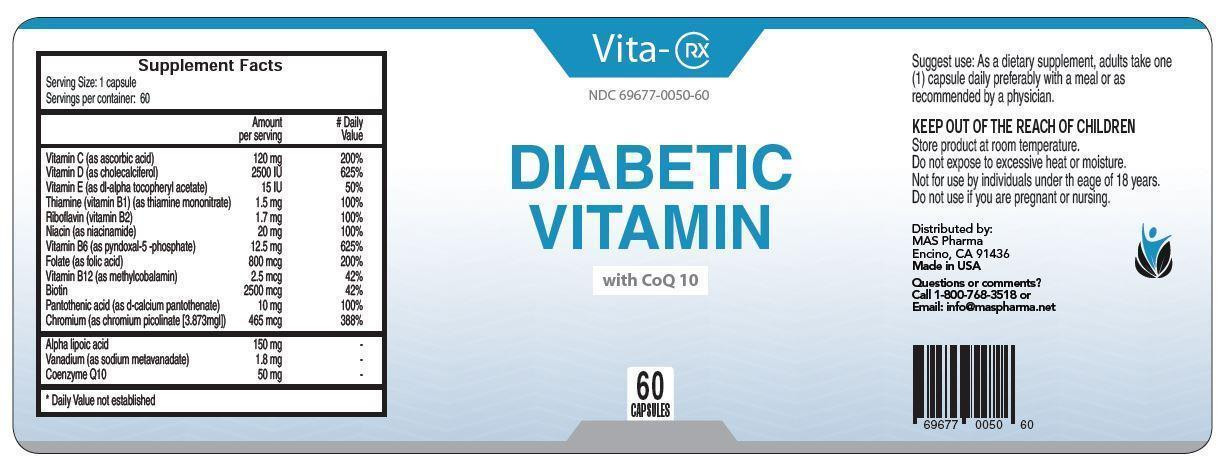
VITA-RX DIABETIC VITAMIN
vitamin c, vitamin d, vitamin e, thiamine, riboflavin, niacin, vitamin b6, folate, vitamin b12, biotin, pantothenic acid, chromium capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65121-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 0.0625 mg .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 10 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE MONONITRATE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 20 mg PYRIDOXAL PHOSPHATE ANHYDROUS (UNII: F06SGE49M6) (PYRIDOXAL PHOSPHATE ANHYDROUS - UNII:F06SGE49M6) PYRIDOXAL PHOSPHATE ANHYDROUS 12.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 0.8 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 0.0025 mg BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 2.5 mg CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 10 mg CHROMIUM PICOLINATE (UNII: S71T8B8Z6P) (CHROMIC CATION - UNII:X1N4508KF1) CHROMIC CATION 0.465 mg THIOCTIC ACID (UNII: 73Y7P0K73Y) (THIOCTIC ACID - UNII:73Y7P0K73Y) THIOCTIC ACID 150 mg UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 50 mg Inactive Ingredients Ingredient Name Strength SODIUM METAVANADATE TETRAHYDRATE (UNII: 2DO8B1WW6L) 1.8 mg GELATIN (UNII: 2G86QN327L) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (clear) Score no score Shape CAPSULE Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65121-301-01 60 in 1 PACKAGE; Type 0: Not a Combination Product 08/11/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/11/2015 Labeler - Pure Source, LLC (080354456) Establishment Name Address ID/FEI Business Operations Pure Source, LLC 080354456 manufacture(65121-301)