| NDC | 69543-340-30 |
| Set ID | 6998a356-6a0d-4cc6-8719-a9b52c8799b6 |
| Category | DIETARY SUPPLEMENT |
| Packager | Virtus Pharmaceuticals |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
HEALTH CLAIM
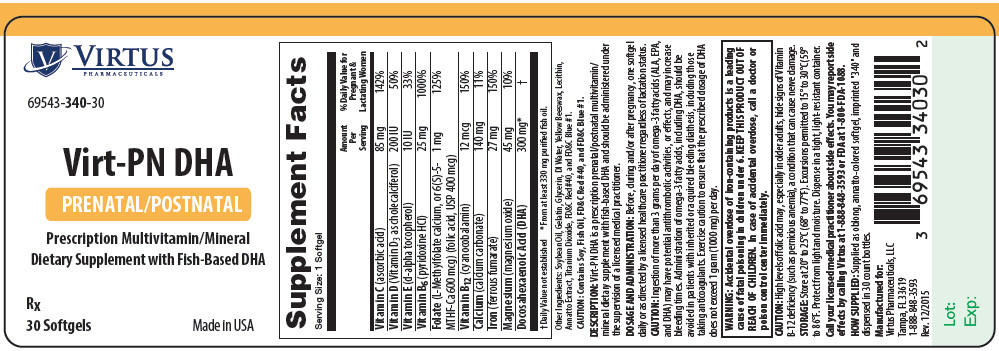
Supplement Facts Serving Size: 1 Softgel Amount Per Serving % Daily Value for Pregnant and Lactating Women Vitamin C (ascorbic acid) 85 mg 142% Vitamin D (Vitamin D3 as cholecalciferol) 200 IU 50% Vitamin E (d-alpha tocopherol) 10 IU 33% Vitamin B6 (pyridoxine HCl) 25 mg 1000% Folate (L-Methylfolate calcium, or 6(S)-5-MTHF-Ca 600 mcg) (folic acid, USP 400 mcg) 1 mg 125% Vitamin B12 (cyanocobalamin) 12 mcg 150% Calcium (calcium carbonate) 140 mg 11% Iron (ferrous fumarate) 27 mg 150% Magnesium (magnesium oxide) 45 mg 10% Docosahexaenoic Acid (DHA) 300 mg* † Other Ingredients: Soybean Oil, Gelatin, Glycerin, DI Water, Yellow Beeswax, Lecithin, Annatto Extract, Titanium Dioxide, FD&C Red #40, and FD&C Blue #1.
CAUTION: Contains Soy, Fish Oil, FD&C Red #40, and FD&C Blue #1.
- DESCRIPTION
- DOSAGE AND ADMINISTRATION
-
CAUTION
Ingestion of more than 3 grams per day of omega-3 fatty acids (ALA, EPA, and DHA) may have potential antithrombotic activities, or effects, and may increase bleeding times. Administration of omega-3 fatty acids, including DHA, should be avoided in patients with inherited or acquired bleeding diathesis, including those taking anticoagulants. Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) per day.
- WARNINGS
- CAUTION
- SAFE HANDLING WARNING
- HEALTH CLAIM
- HOW SUPPLIED
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 30 Softgel Bottle Label
-
INGREDIENTS AND APPEARANCE
VIRT-PN DHA
ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, pyridoxine hydrochloride, levomefolate calcium, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, magnesium oxide, and doconexent capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69543-340 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 85 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 200 [iU] .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 10 [iU] PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 600 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 400 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 140 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 45 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 300 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) SOYBEAN OIL (UNII: 241ATL177A) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) ANNATTO (UNII: 6PQP1V1B6O) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69543-340-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 09/08/2015 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 25 mm imprint Labeler - Virtus Pharmaceuticals (079659493)