| NDC | 37000-034-24, 37000-550-12, 37000-565-12 |
| Set ID | 416dd077-686a-320c-e054-00144ff8d46c |
| Category | HUMAN OTC DRUG LABEL |
| Packager | The Procter & Gamble Manufacturing Company |
| Generic Name | |
| Product Class | Antihistamine |
| Product Number | |
| Application Number | PART341 |
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 30 mL)
- Uses
-
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- glaucoma
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
- trouble urinating due to enlarged prostate gland
- a sodium-restricted diet
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 15 mL)
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions?
-
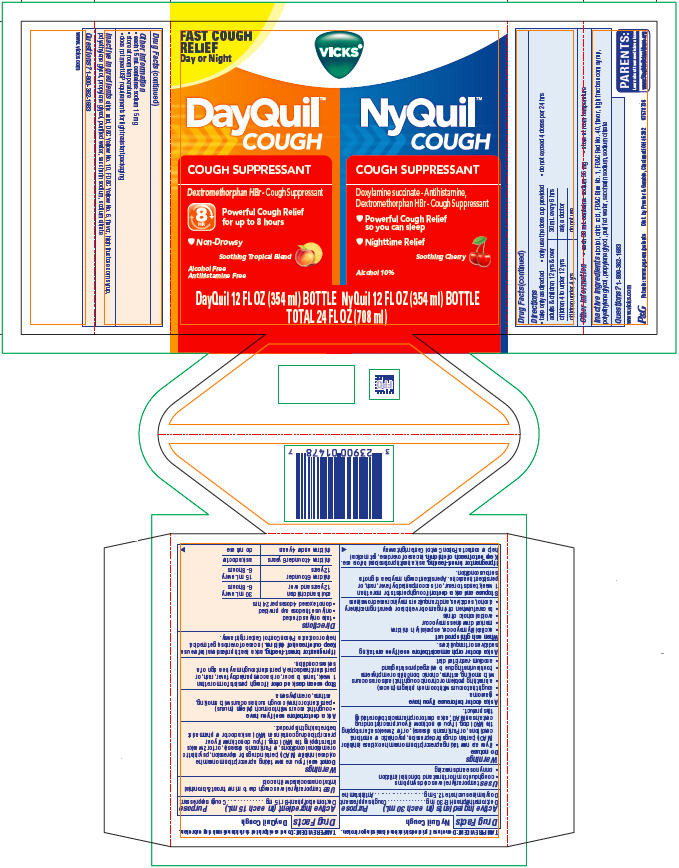
PRINCIPAL DISPLAY PANEL - Kit Carton
FAST COUGH
RELIEF
Day or Night
VICKS ®
DayQuil ™
COUGH
COUGH SUPPRESSANT
Dextromethorphan HBr- Cough Suppressant
8 HR
Powerful Cough Relief
for up to 8 hours
- Non-Drowsy
Soothing Tropical Blend
Alcohol Free
Antihistamine Free
DayQuil 12 FL OZ (354 ml) BOTTLE
NyQuil ™
COUGH
COUGH SUPPRESSANT
Doxylamine Succinate - Antihistamine
Dextromethorphan HBr - Cough Suppressant
- Powerful Cough Relief so you can sleep
- Nighttime Relief
Soothing Cherry
Alcohol 10%
NyQuil 12 FL OZ (354 ml) BOTTLE
TOTAL 24 FL OZ (708 ml)

-
INGREDIENTS AND APPEARANCE
VICKS DAYQUIL TROPICAL AND VICKS NYQUIL CHERRY COUGH
acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride, and doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-034 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-034-24 1 in 1 PACKAGE; Type 0: Not a Combination Product 08/01/2013 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 354 mL Part 2 1 BOTTLE, PLASTIC 354 mL Part 1 of 2 VICKS DAYQUIL COUGH
dextromethorphan hydrobromide liquidProduct Information Item Code (Source) NDC:37000-565 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg in 15 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM CITRATE (UNII: 1Q73Q2JULR) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color orange Score Shape Size Flavor PINEAPPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-565-12 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/22/2011 Part 2 of 2 VICKS NYQUIL COUGH
dextromethorphan hydrobromide and doxylamine succinate liquidProduct Information Item Code (Source) NDC:37000-550 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 30 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 30 mL Inactive Ingredients Ingredient Name Strength HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) ALCOHOL (UNII: 3K9958V90M) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM CITRATE (UNII: 1Q73Q2JULR) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-550-12 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/22/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2013 Labeler - The Procter & Gamble Manufacturing Company (004238200)