| NDC | 57955-0066-2 |
| Set ID | 4276dc69-d045-4bb7-99df-0df6476fe44e |
| Category | HUMAN OTC DRUG LABEL |
| Packager | King Bio Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Drug Facts__________________________________________________________________________________________________________
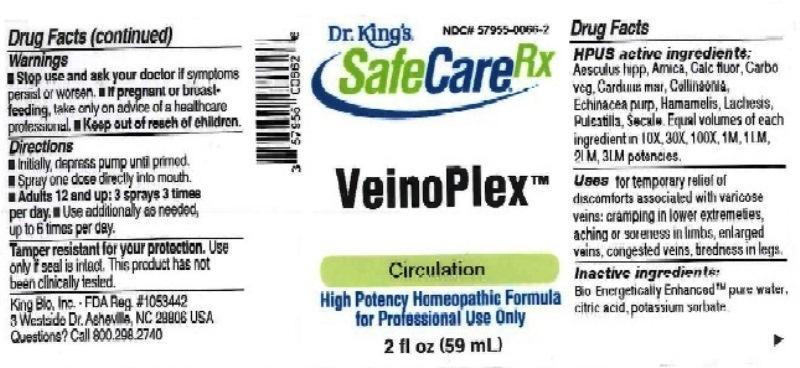
HPUS active ingredients: Aesculus hippocastanum, Arnica montana, Calcarea fluorica, Carbo vegetabilis, Carduus marianus, Collinsonia canadensis, Echinacea purpurea, Hamamelis virginiana, Lachesis mutus, Pulsatilla, Secale cornutum. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, 1LM, 2LM, 3LM potencies.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VEINOPLEX
aesculus hippocastanum, arnica montana, calcarea fluorica, carbo vegetabilis, carduus marianus, collinsonia canadensis, echinacea purpurea, hamamelis virginiana, lachesis mutus, pulsatilla, secale cornutum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0066 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 10 [hp_X] in 59 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 10 [hp_X] in 59 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 10 [hp_X] in 59 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 59 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 10 [hp_X] in 59 mL COLLINSONIA CANADENSIS ROOT (UNII: O2630F3XDR) (COLLINSONIA CANADENSIS ROOT - UNII:O2630F3XDR) COLLINSONIA CANADENSIS ROOT 10 [hp_X] in 59 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 10 [hp_X] in 59 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 10 [hp_X] in 59 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 10 [hp_X] in 59 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 10 [hp_X] in 59 mL CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0066-2 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 10/22/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/22/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0066)