| NDC | 52731-7045-1, 52731-7045-2 |
| Set ID | 9fa9b70a-72ce-4677-b8da-41d213fa79e4 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Nova Homeopathic Therapeutics, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Purpose:
- Usage and Dosage:
- DOSAGE & ADMINISTRATION
- Warnings:
- WARNINGS
- Active Ingredients:
- Inactive Ingredients:
- QUESTIONS
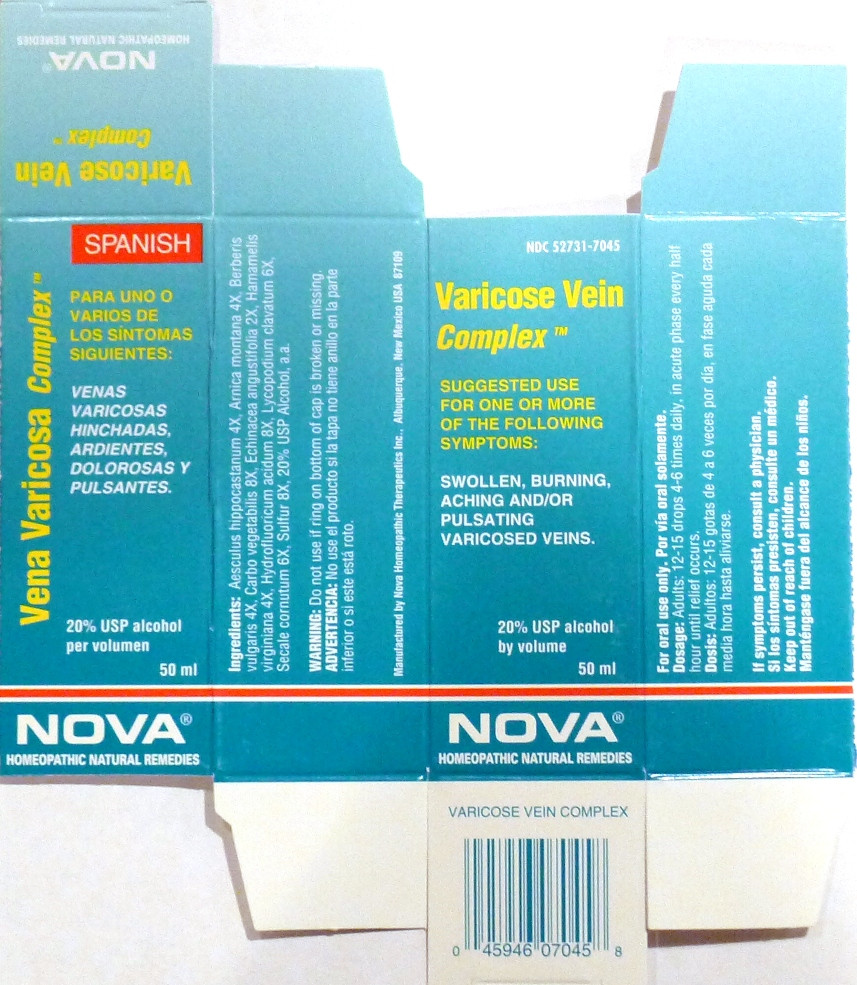
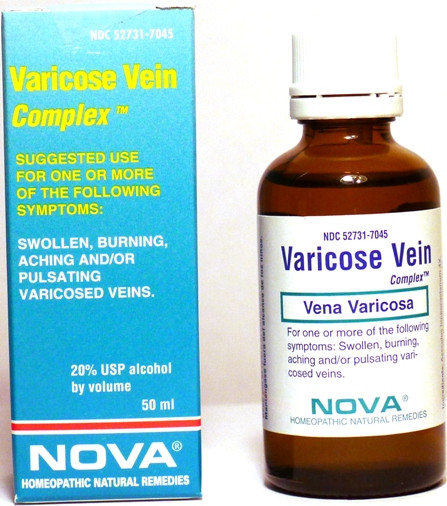
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VARICOSE VEIN COMPLEX
aesculus hippocastanum, arnica montana, berberis vulgaris, carbo vegetabilis, echinacea angustifolia, hamamelis virginiana, hydrofluoricum acidum, lycopodium clavatum, secale cornutum, sulfur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52731-7045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 4 [hp_X] in 1 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 4 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 8 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 2 [hp_X] in 1 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 4 [hp_X] in 1 mL HYDROFLUORIC ACID (UNII: RGL5YE86CZ) (HYDROFLUORIC ACID - UNII:RGL5YE86CZ) HYDROFLUORIC ACID 8 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_X] in 1 mL CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 6 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 8 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52731-7045-2 1 in 1 BOX 1 NDC:52731-7045-1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2011 Labeler - Nova Homeopathic Therapeutics, Inc. (194394540) Registrant - Nova Homeopathic Therapeutics, Inc. (194394540) Establishment Name Address ID/FEI Business Operations Nova Homeopathic Therapeutics, Inc. 194394540 manufacture, label, pack