| NDC | 49304-0010-1 |
| Set ID | af5bd48c-1e1e-42b8-918f-50ce3db129ff |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Alternative Pharmacy |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
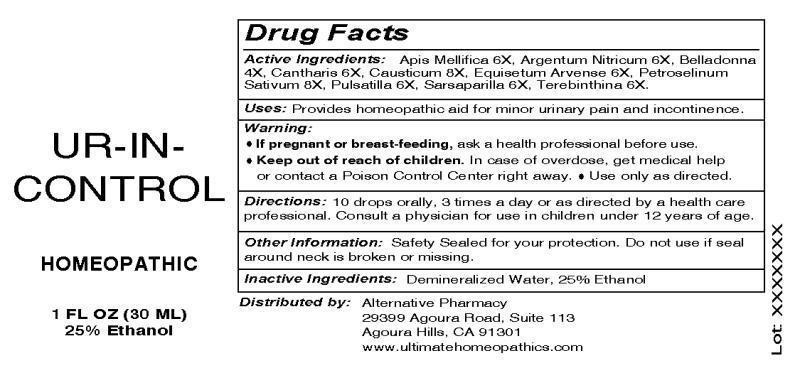
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- KEEP OUT OF REACH OF CHILDREN:
- INDICATIONS:
- QUESTIONS:
- PACKAGE DISPLAY LABEL:
-
INGREDIENTS AND APPEARANCE
UR IN CONTROL
apis mellifica, argentum nitricum, belladonna, cantharis, causticum, equsetum arvense, petroselinum sativum, pulsatilla, sarsaparilla, terebinthina liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49304-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 1 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 6 [hp_X] in 1 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 4 [hp_X] in 1 mL LYTTA VESICATORIA (UNII: 3Q034RO3BT) (LYTTA VESICATORIA - UNII:3Q034RO3BT) LYTTA VESICATORIA 6 [hp_X] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 8 [hp_X] in 1 mL EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) (EQUISETUM ARVENSE TOP - UNII:1DP6Y6B65Z) EQUISETUM ARVENSE TOP 6 [hp_X] in 1 mL PETROSELINUM CRISPUM (UNII: 1WZA4Y92EX) (PETROSELINUM CRISPUM - UNII:1WZA4Y92EX) PETROSELINUM CRISPUM 8 [hp_X] in 1 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_X] in 1 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 6 [hp_X] in 1 mL TURPENTINE OIL (UNII: C5H0QJ6V7F) (TURPENTINE OIL - UNII:C5H0QJ6V7F) TURPENTINE OIL 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49304-0010-1 30 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/21/2013 Labeler - Alternative Pharmacy (139900554) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(49304-0010) , api manufacture(49304-0010) , label(49304-0010) , pack(49304-0010)