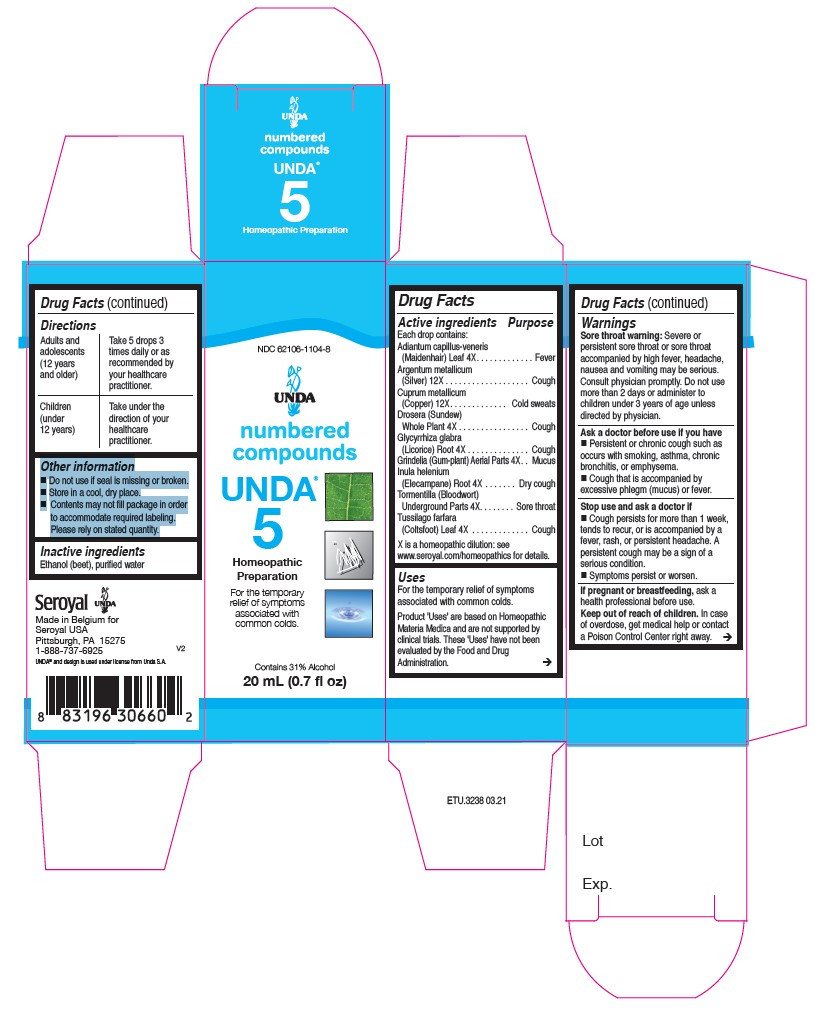
| NDC | 62106-1104-8, 62106-1107-8, 62106-1113-8, 62106-1155-8, 62106-1158-8, 62106-1165-8 |
| Set ID | 1789ed21-3bc9-3b86-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
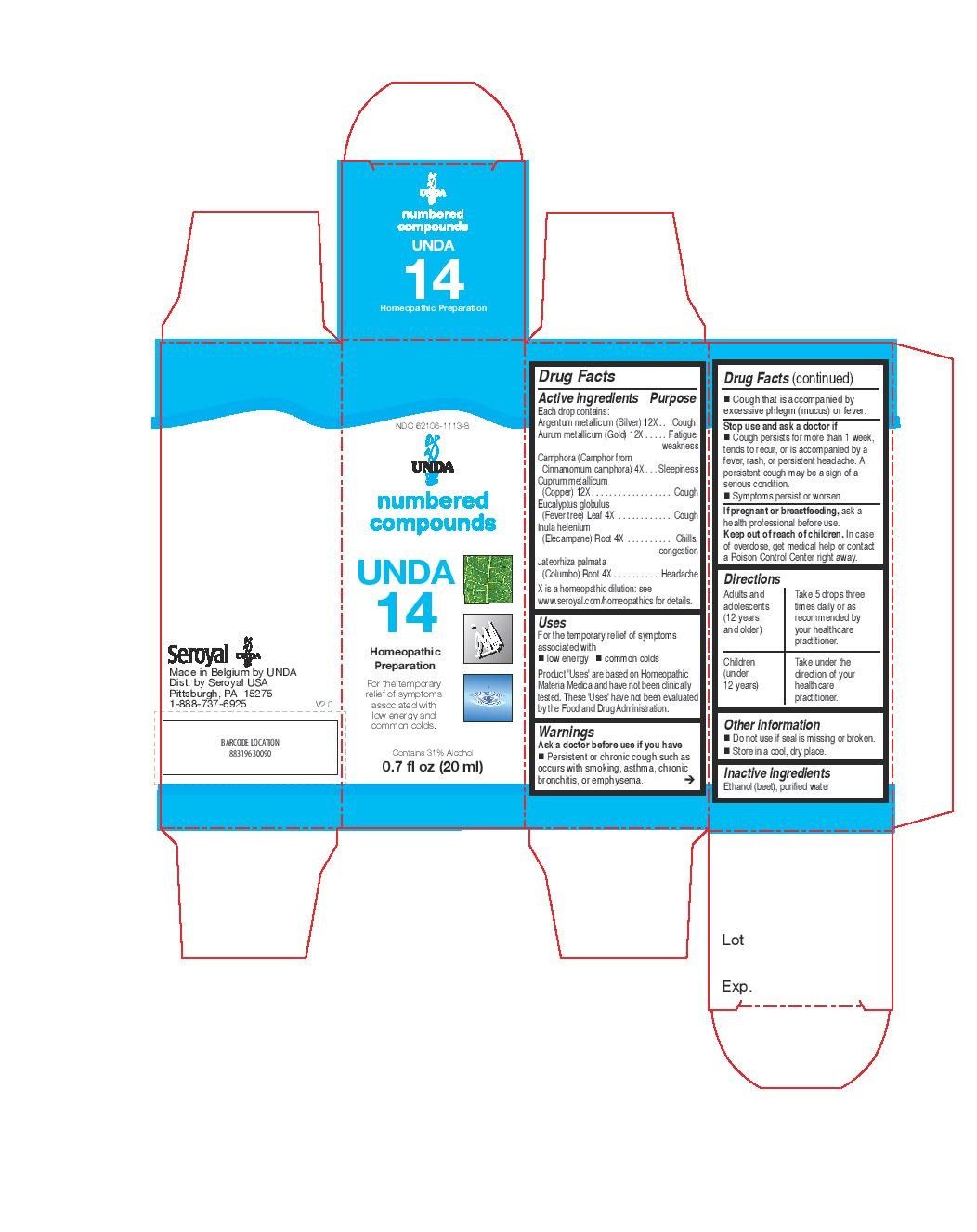
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Tormentilla (Bloodwort) Rhizome . 4X
Tussilago farfara (Coltsfoot) Whole Plant . 4X
Adiantum capillus-veneris (Maidenhair) Leaf. 4X
Glycyrrhiza glabra (Licorice) Root . 4X
Drosera (Sundew)Whole Plant . 4X
Grindelia Aerial Parts. 4X
Inula helenium (Elecampane) Root . 4X
Cuprum metallicum(Copper) . 12X
Argentum metallicum(Silver). 12X - PURPOSE
-
WARNINGS
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache,
nausea and vomiting may be serious.
Consult physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by physician.Ask a doctor before use if you have Persistent or chronic cough such as occurs with smoking, asthma, chronic
bronchitis, or emphysema. Cough that is accompanied by excessive phlegm (mucus) or fever.Stop use and ask a doctor if Cough persists for more than 1 week, tends to recur, or is accompanied by a
fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
And if symptoms persist or worsen.If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.In case of overdose, get medical help or contact a Poison Control Center right away.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
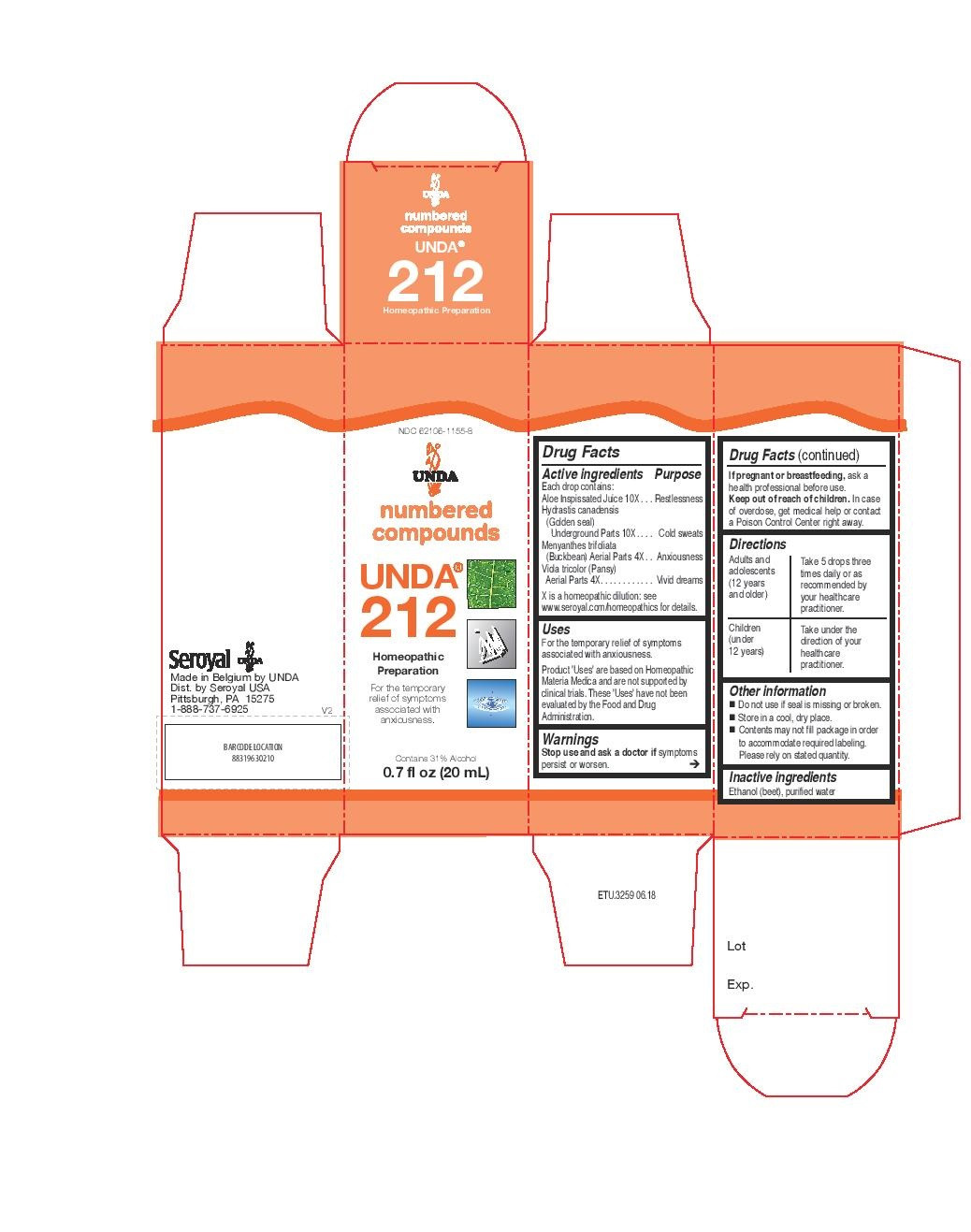
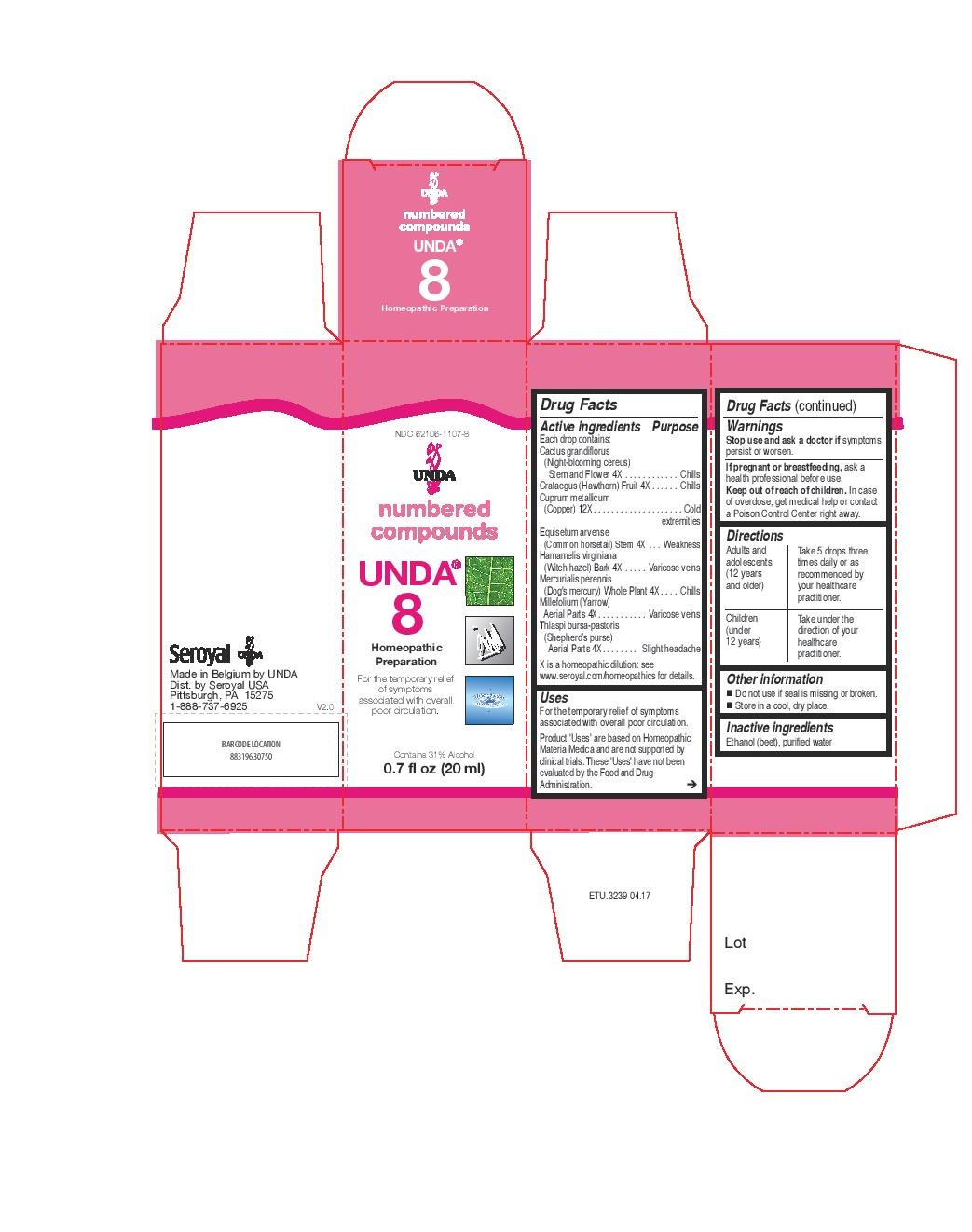
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Cactus grandiflorus (Night-blooming cereus) Stem and Flower 4X
Crataegus (Hawthorn) Fruit 4X
Cuprum metallicum (Copper) 12X
Equisetum arvense (Common horsetail) Stem 4X
Hamamelis virginiana (Witch hazel) Bark 4X
Mercurialis perennis (Dog's mercury) Whole Plant 4X
Millefolium (Yarrow) Aerial Parts 4X
Thlaspi bursa-pastoris (Shepherd’s purse) Aerial Parts 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- STOP USE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with overall poor circulation.Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- OTHER SAFETY INFORMATION
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you have
Persistent or chronic cough such as
occurs with smoking, asthma, chronic
bronchitis, or emphysema.Cough that is accompanied by
excessive phlegm (mucus) or fever.
Stop use and ask a doctor if
Cough persists for more than 1 week,
tends to recur, or is accompanied by a
fever, rash, or persistent headache. A
persistent cough may be a sign of a
serious condition.
Symptoms persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- STOP USE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms
associated with low energy and common coldsDirections
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - OTHER SAFETY INFORMATION
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- STOP USE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
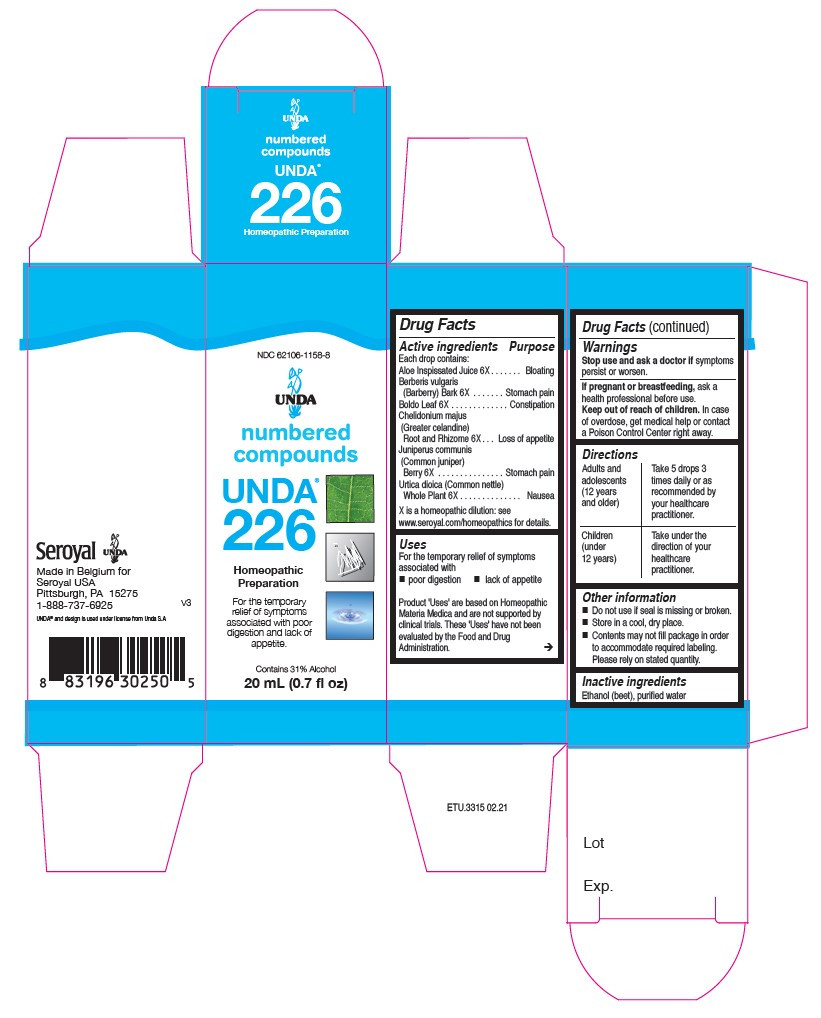
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with poor digestion and lack of appetite.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - OTHER SAFETY INFORMATION
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with
poor digestion
minor urinary discomfortDirections
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 8
hamamelis virginiana, millefolium, equisetum arvense, mercurialis perennis, thlaspi bursa-pastoris, cactus grandiflorus, crataegus, cuprum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) (HAMAMELIS VIRGINIANA BARK - UNII:IH3063S9MY) HAMAMELIS VIRGINIANA BARK 4 [hp_X] in 20 mL MERCURIALIS PERENNIS (UNII: Q35465A1MA) (MERCURIALIS PERENNIS - UNII:Q35465A1MA) MERCURIALIS PERENNIS 4 [hp_X] in 20 mL CRATAEGUS FRUIT (UNII: Q21UUL2105) (CRATAEGUS FRUIT - UNII:Q21UUL2105) CRATAEGUS FRUIT 4 [hp_X] in 20 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 20 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 4 [hp_X] in 20 mL EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) (EQUISETUM ARVENSE BRANCH - UNII:1L0VKZ185E) EQUISETUM ARVENSE BRANCH 4 [hp_X] in 20 mL CAPSELLA BURSA-PASTORIS (UNII: W0X9457M59) (CAPSELLA BURSA-PASTORIS - UNII:W0X9457M59) CAPSELLA BURSA-PASTORIS 4 [hp_X] in 20 mL SELENICEREUS GRANDIFLORUS STEM (UNII: 7114SV0MYK) (SELENICEREUS GRANDIFLORUS STEM - UNII:7114SV0MYK) SELENICEREUS GRANDIFLORUS STEM 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1107-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 UNDA 5
tormentilla, tussilago farfara, adiantum capillus-veneris, glycyrrhiza glabra, drosera, grindelia, inula helenium, cuprum metallicum, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTENTILLA ERECTA ROOT (UNII: BI896CKT6B) (POTENTILLA ERECTA ROOT - UNII:BI896CKT6B) POTENTILLA ERECTA ROOT 4 [hp_X] in 20 mL ADIANTUM CAPILLUS-VENERIS LEAF (UNII: 4817H7M565) (ADIANTUM CAPILLUS-VENERIS LEAF - UNII:4817H7M565) ADIANTUM CAPILLUS-VENERIS LEAF 4 [hp_X] in 20 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 4 [hp_X] in 20 mL GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 4 [hp_X] in 20 mL GRINDELIA HIRSUTULA FLOWERING TOP (UNII: IDB0NAZ6AI) (GRINDELIA HIRSUTULA FLOWERING TOP - UNII:IDB0NAZ6AI) GRINDELIA HIRSUTULA FLOWERING TOP 4 [hp_X] in 20 mL INULA HELENIUM ROOT (UNII: E55SMD6DA8) (INULA HELENIUM ROOT - UNII:E55SMD6DA8) INULA HELENIUM ROOT 4 [hp_X] in 20 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL TUSSILAGO FARFARA WHOLE (UNII: 6177A89GA2) (TUSSILAGO FARFARA WHOLE - UNII:6177A89GA2) TUSSILAGO FARFARA WHOLE 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1104-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 UNDA 14
eucalyptus globulus, inula helenium, jateorhiza palmata, camphora, argentum metallicum, aurum metallicum, cuprum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INULA HELENIUM ROOT (UNII: E55SMD6DA8) (INULA HELENIUM ROOT - UNII:E55SMD6DA8) INULA HELENIUM ROOT 4 [hp_X] in 20 mL JATEORHIZA CALUMBA ROOT (UNII: V36I2B8LD5) (JATEORHIZA CALUMBA ROOT - UNII:V36I2B8LD5) JATEORHIZA CALUMBA ROOT 4 [hp_X] in 20 mL CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 20 mL EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1113-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 UNDA 212
menyanthes trifoliata, viola tricolor, hydrastis canadensis, aloe socotrina liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENYANTHES TRIFOLIATA (UNII: 7H0QTZ446K) (MENYANTHES TRIFOLIATA - UNII:7H0QTZ446K) MENYANTHES TRIFOLIATA 4 [hp_X] in 20 mL VIOLA TRICOLOR (UNII: 9Q24RAI43V) (VIOLA TRICOLOR - UNII:9Q24RAI43V) VIOLA TRICOLOR 4 [hp_X] in 20 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 10 [hp_X] in 20 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 10 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1155-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 UNDA 226
urtica dioica, berberis vulgaris, boldo, juniperus communis, aloe socotrina, chelidonium majus liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1158 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 6 [hp_X] in 20 mL URTICA DIOICA (UNII: 710FLW4U46) (URTICA DIOICA - UNII:710FLW4U46) URTICA DIOICA 6 [hp_X] in 20 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 6 [hp_X] in 20 mL JUNIPER BERRY (UNII: O84B5194RL) (JUNIPER BERRY - UNII:O84B5194RL) JUNIPER BERRY 6 [hp_X] in 20 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 6 [hp_X] in 20 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 6 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1158-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 UNDA 258
berberis vulgaris, bryonia dioica, lycopodium clavatum, mercurialis annua, hydrastis canadensis, boldo, solidago virgaurea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1165 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 20 mL MERCURIALIS ANNUA FLOWERING TOP (UNII: 7D28M9SL9B) (MERCURIALIS ANNUA FLOWERING TOP - UNII:7D28M9SL9B) MERCURIALIS ANNUA FLOWERING TOP 4 [hp_X] in 20 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 4 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 4 [hp_X] in 20 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_X] in 20 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1165-8 1 in 1 CARTON 06/02/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1104, 62106-1107, 62106-1113, 62106-1155, 62106-1158, 62106-1165)