| NDC | 62106-1101-8, 62106-1105-8, 62106-1108-8, 62106-1168-8, 62106-1174-8 |
| Set ID | 079c945e-7853-3410-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Asparagus officinalis (Asparagus) Shoot 4X
Berberis vulgaris (Barberry) Bark 4X
Genista tinctoria Aerial Parts 4X
Juniperus communis (Common juniper) Berry 4X
Petroselinum sativum (Parsley) Whole Plant 4X
Rhamnus frangula (Buckthorn) Bark 4X
Thlaspi bursa-pastoris (Shepherd’s purse) Aerial Parts 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
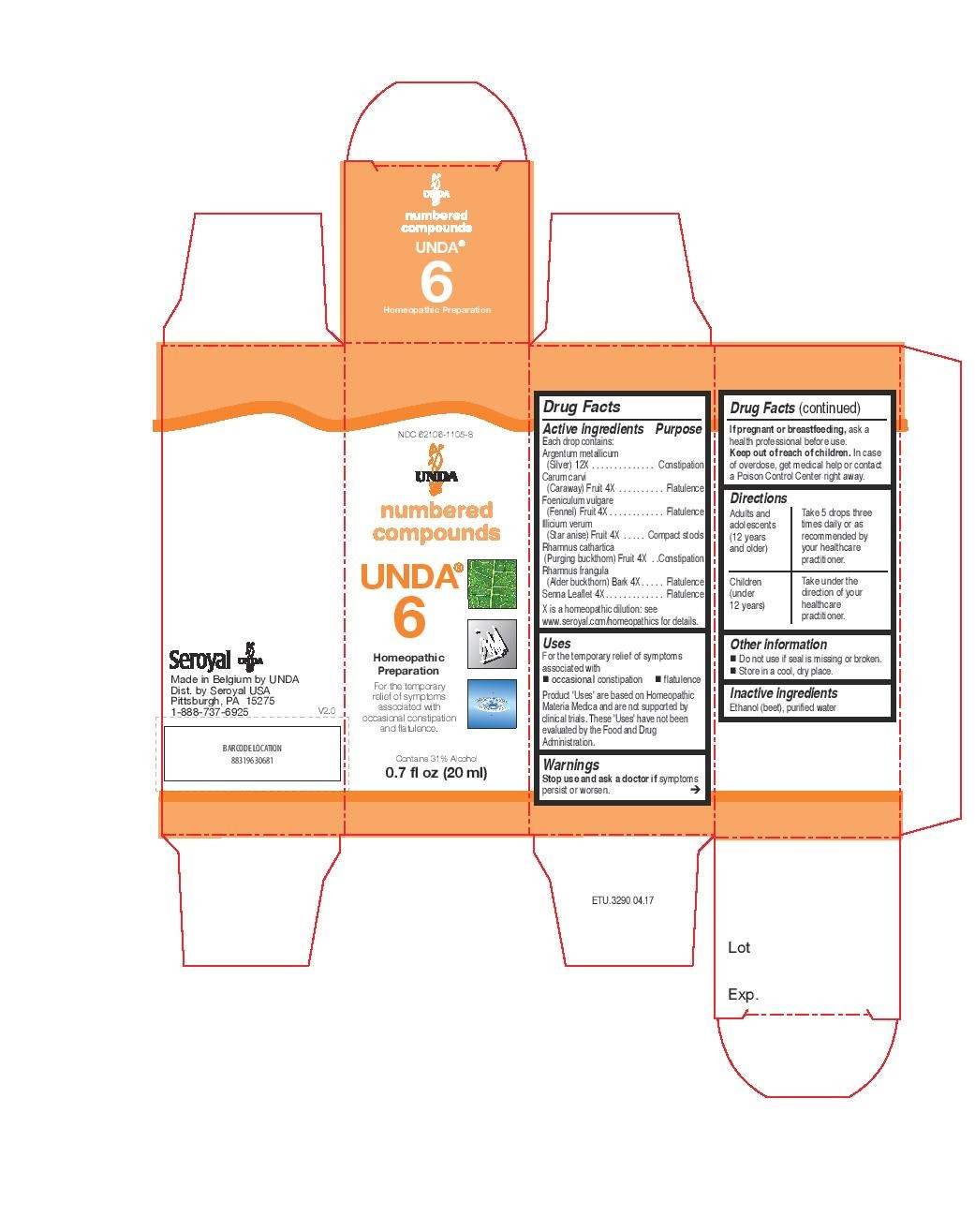
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with occasional constipation and flatulence.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
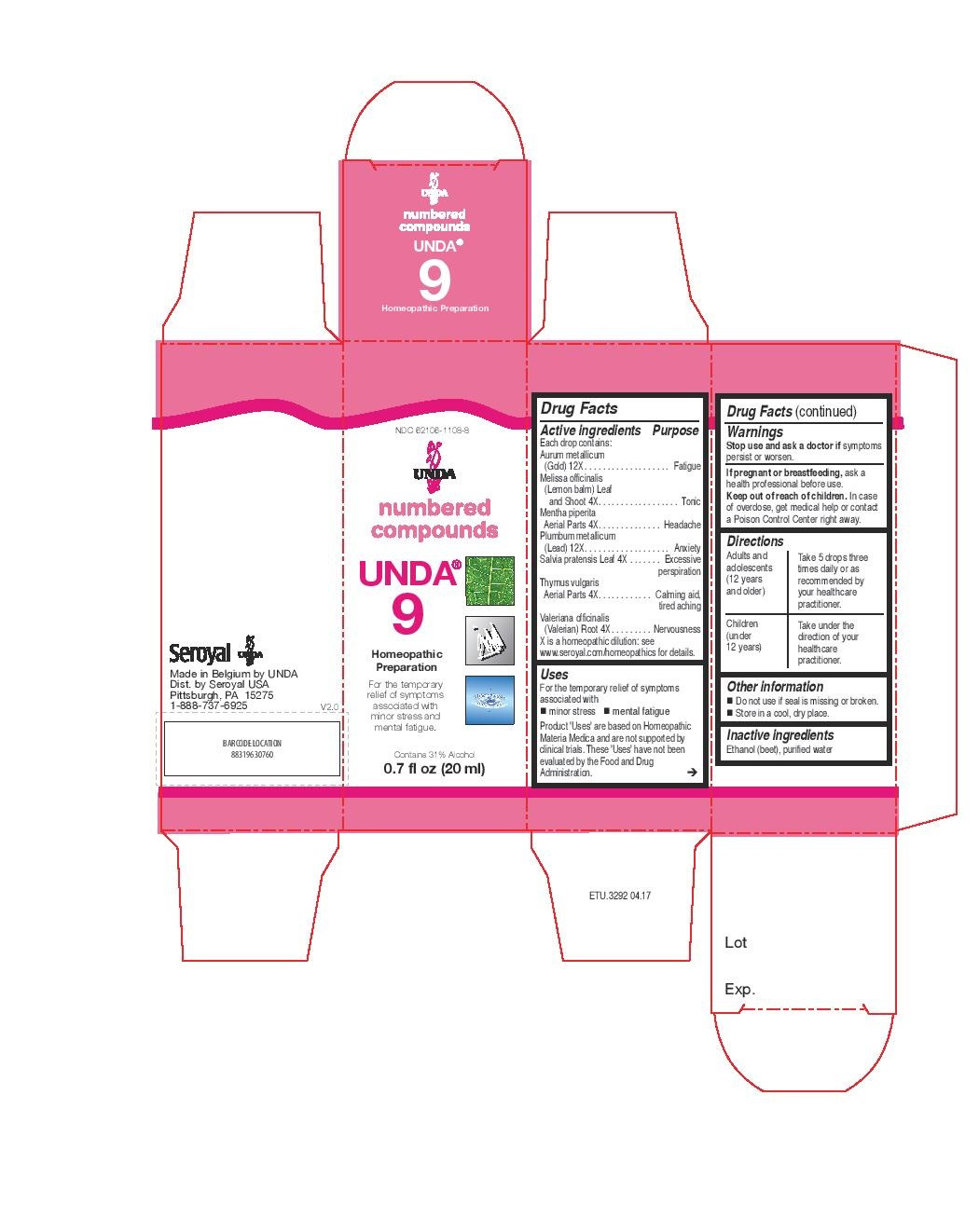
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with minor stress and mental fatigue.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
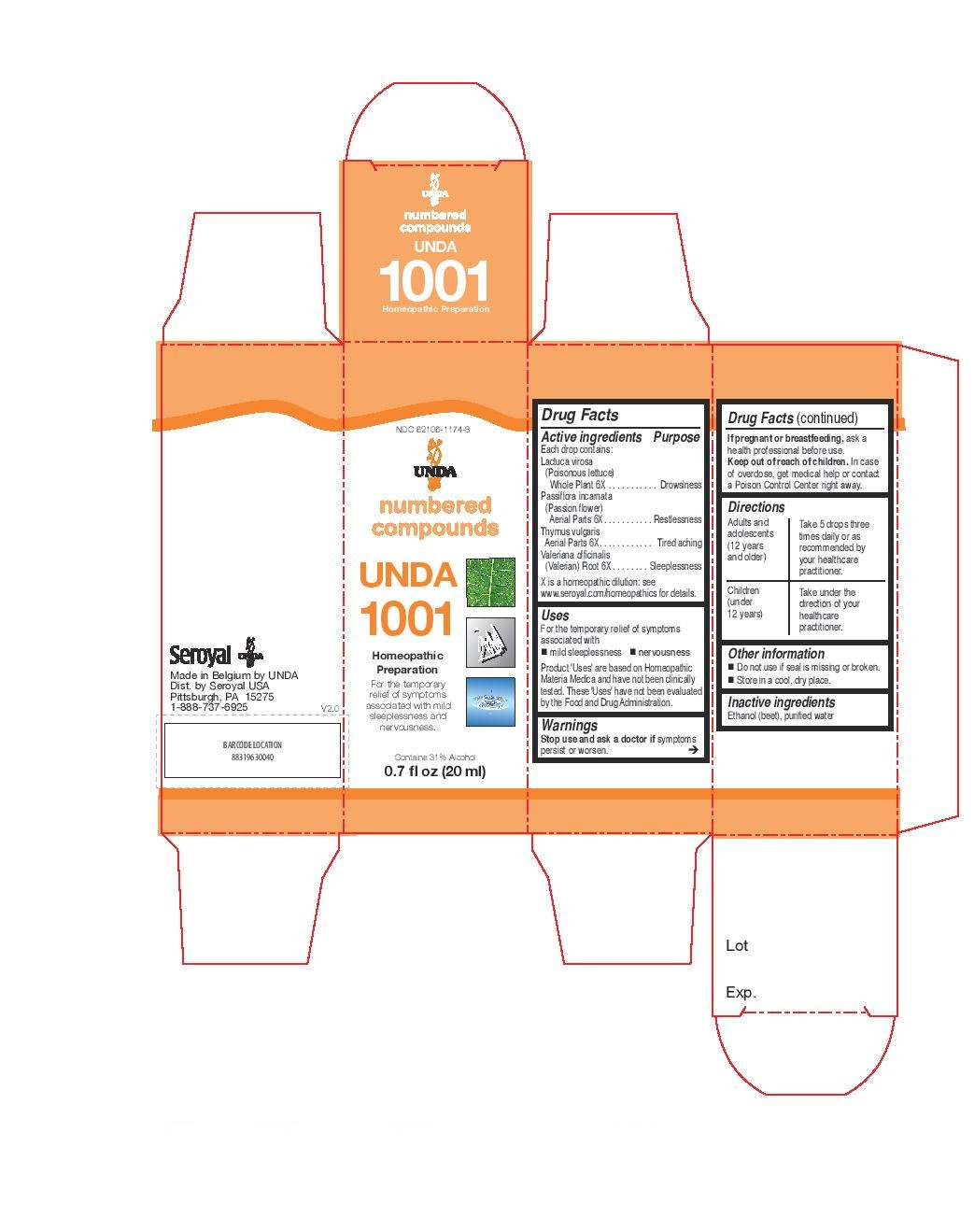
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms
associated with mild sleeplessness and nervousness.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
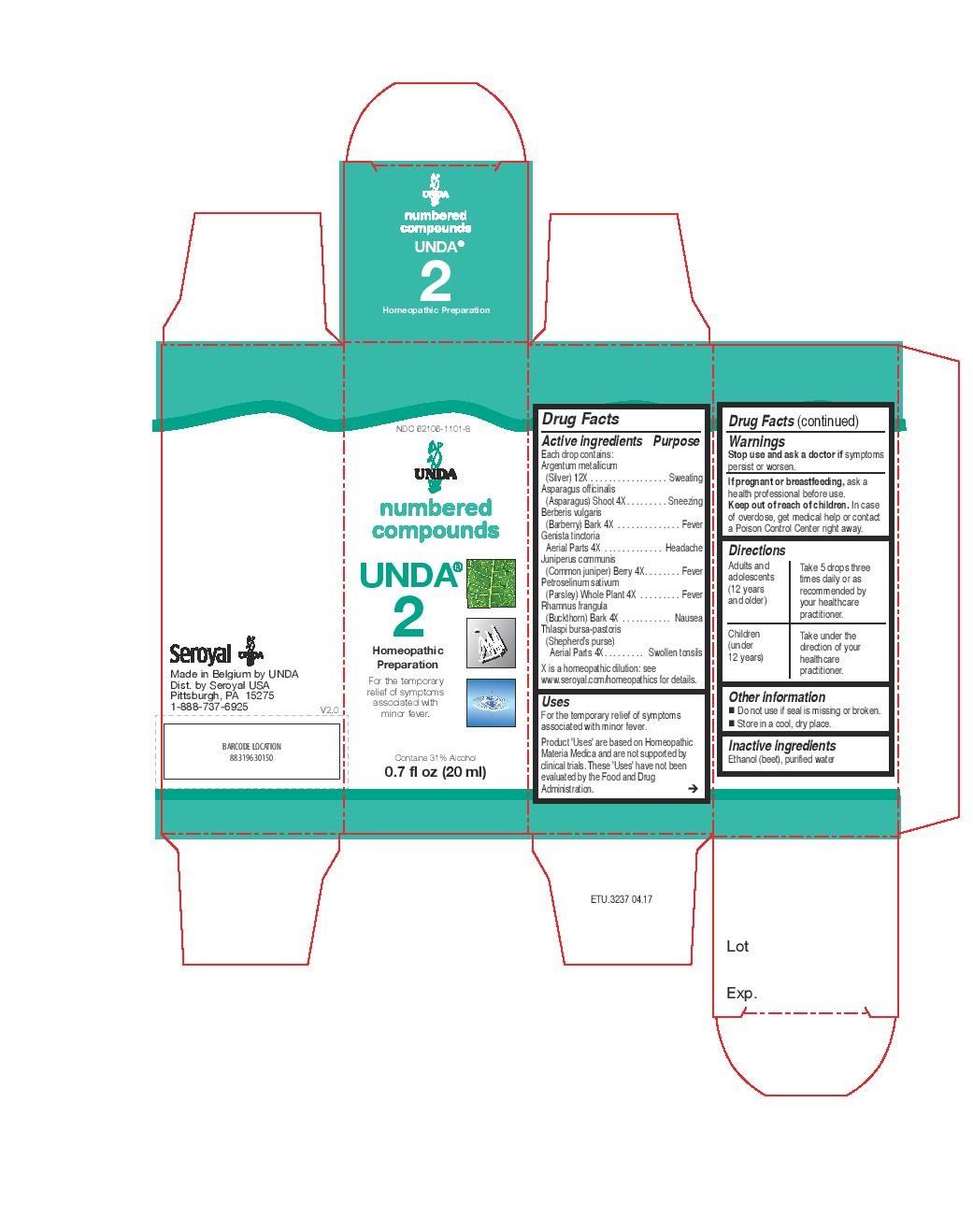
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 6
senna leaflet, rhamnus frangula bark, carum carvi, foeniculum vulgare, illicium verum, rhamnus cathartica, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 4 [hp_X] in 20 mL FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 4 [hp_X] in 20 mL CARAWAY SEED (UNII: W2FH8O2BBE) (CARAWAY SEED - UNII:W2FH8O2BBE) CARAWAY SEED 4 [hp_X] in 20 mL FENNEL SEED (UNII: G3QC02NIE6) (FENNEL SEED - UNII:G3QC02NIE6) FENNEL SEED 4 [hp_X] in 20 mL ILLICIUM VERUM WHOLE (UNII: 52JDS841PX) (ILLICIUM VERUM WHOLE - UNII:52JDS841PX) ILLICIUM VERUM WHOLE 4 [hp_X] in 20 mL RHAMNUS CATHARTICA FRUIT (UNII: B4R1EZ1R1D) (RHAMNUS CATHARTICA FRUIT - UNII:B4R1EZ1R1D) RHAMNUS CATHARTICA FRUIT 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1105-8 1 in 1 CARTON 05/26/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/26/2015 UNDA 2
petroselinum sativum, rhamnus frangula, thlaspi bursa-pastoris, juniperus communis, asparagus officinalis, berberis vulgaris, genista tinctoria, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_X] in 20 mL PETROSELINUM CRISPUM (UNII: 1WZA4Y92EX) (PETROSELINUM CRISPUM - UNII:1WZA4Y92EX) PETROSELINUM CRISPUM 4 [hp_X] in 20 mL ASPARAGUS (UNII: Z1EJP3037Z) (ASPARAGUS - UNII:Z1EJP3037Z) ASPARAGUS 4 [hp_X] in 20 mL FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 4 [hp_X] in 20 mL CAPSELLA BURSA-PASTORIS (UNII: W0X9457M59) (CAPSELLA BURSA-PASTORIS - UNII:W0X9457M59) CAPSELLA BURSA-PASTORIS 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL GENISTA TINCTORIA (UNII: 1F26HNB59H) (GENISTA TINCTORIA - UNII:1F26HNB59H) GENISTA TINCTORIA 4 [hp_X] in 20 mL JUNIPER BERRY (UNII: O84B5194RL) (JUNIPER BERRY - UNII:O84B5194RL) JUNIPER BERRY 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1101-8 1 in 1 CARTON 05/26/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/26/2015 UNDA 295
pulsatilla, nux vomica, aloe socotrina liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1168 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 4 [hp_X] in 20 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 6 [hp_X] in 20 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1168-8 1 in 1 CARTON 05/27/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2015 UNDA 9
mentha piperita, melissa officinalis, salvia pratensis, thymus vulgaris, valeriana officinalis, aurum metallicum, plumbum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL THYMUS VULGARIS WHOLE (UNII: 8L72OKJ7II) (THYMUS VULGARIS WHOLE - UNII:8L72OKJ7II) THYMUS VULGARIS WHOLE 4 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 12 [hp_X] in 20 mL MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 4 [hp_X] in 20 mL MELISSA OFFICINALIS (UNII: YF70189L0N) (MELISSA OFFICINALIS - UNII:YF70189L0N) MELISSA OFFICINALIS 4 [hp_X] in 20 mL SALVIA PRATENSIS LEAF (UNII: X621621QQV) (SALVIA PRATENSIS LEAF - UNII:X621621QQV) SALVIA PRATENSIS LEAF 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1108-8 1 in 1 CARTON 05/27/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2015 UNDA 1001
thymus vulgaris, valeriana officinalis, lactuca virosa, passiflora incarnata liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1174 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THYMUS VULGARIS WHOLE (UNII: 8L72OKJ7II) (THYMUS VULGARIS WHOLE - UNII:8L72OKJ7II) THYMUS VULGARIS WHOLE 6 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 6 [hp_X] in 20 mL PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 6 [hp_X] in 20 mL LACTUCA VIROSA (UNII: 6D74QW4H67) (LACTUCA VIROSA - UNII:6D74QW4H67) LACTUCA VIROSA 6 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1174-8 1 in 1 CARTON 05/27/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1101, 62106-1105, 62106-1108, 62106-1168, 62106-1174)