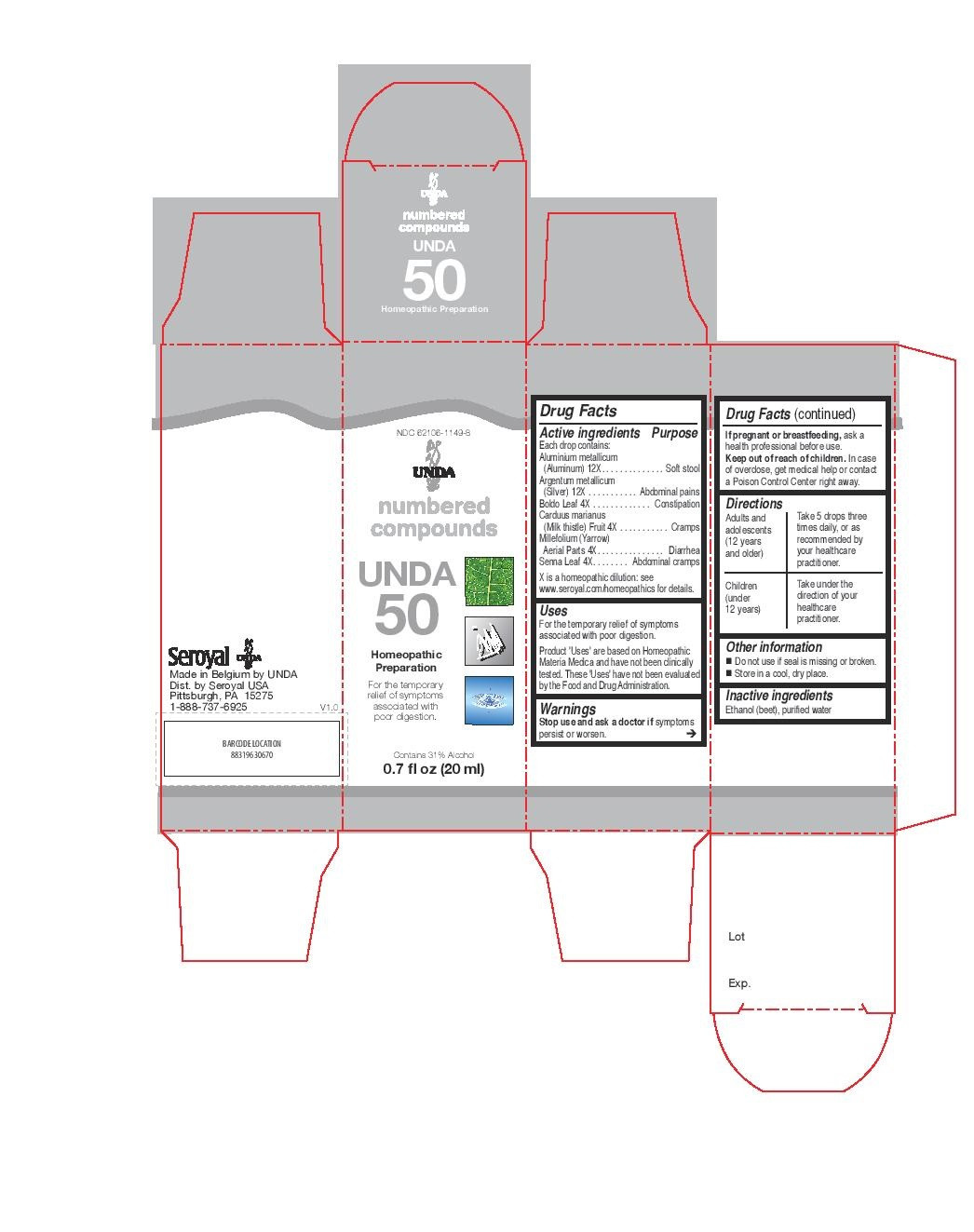
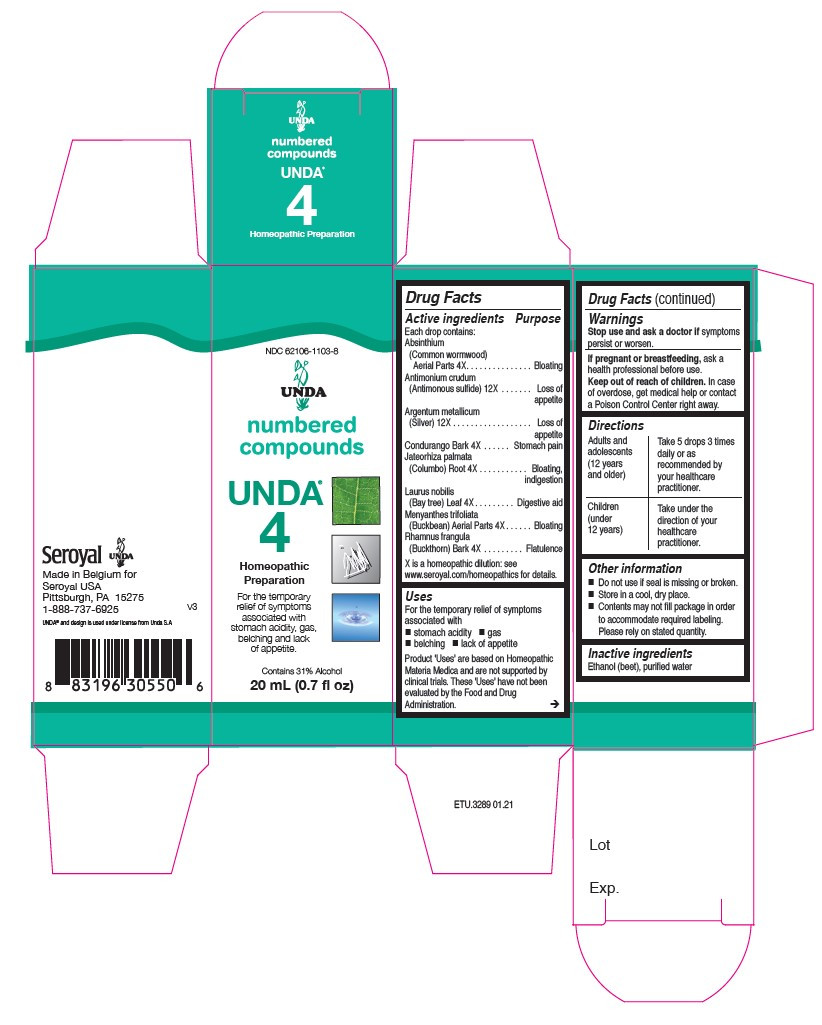
| NDC | 62106-1103-8, 62106-1149-8 |
| Set ID | 1777c76b-5329-3c7b-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Absinthium (Common wormwood) Aerial Parts 4X
Antimonium crudum (Antimonous sulfide) 12X
Argentum metallicum (Silver) 12X
Condurango Bark 4X
Jateorhiza palmata (Columbo) Root 4X
Laurus nobilis (Bay tree) Leaf 4X
Menyanthes trifoliata (Buckbean) Aerial Parts 4X
Rhamnus frangula (Buckthorn) Bark 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- STOP USE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with
stomach acidity, gas, belching and lack of appetite.Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 50
boldo leaf, senna leaflet, carduus marianus, millefolium, aluminium metallicum, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1149 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 4 [hp_X] in 20 mL SILYBUM MARIANUM SEED (UNII: U946SH95EE) (SILYBUM MARIANUM SEED - UNII:U946SH95EE) SILYBUM MARIANUM SEED 4 [hp_X] in 20 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 4 [hp_X] in 20 mL ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1149-8 1 in 1 CARTON 06/01/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2015 UNDA 4
laurus nobilis, condurango, rhamnus frangula, menyanthes trifoliata, absinthium, jateorhiza palmata, argentum metallicum, antimonium crudum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 4 [hp_X] in 20 mL MENYANTHES TRIFOLIATA (UNII: 7H0QTZ446K) (MENYANTHES TRIFOLIATA - UNII:7H0QTZ446K) MENYANTHES TRIFOLIATA 4 [hp_X] in 20 mL WORMWOOD (UNII: F84709P2XV) (WORMWOOD - UNII:F84709P2XV) WORMWOOD 4 [hp_X] in 20 mL JATEORHIZA CALUMBA ROOT (UNII: V36I2B8LD5) (JATEORHIZA CALUMBA ROOT - UNII:V36I2B8LD5) JATEORHIZA CALUMBA ROOT 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 12 [hp_X] in 20 mL BAY LEAF (UNII: WS0Y3M85RF) (BAY LEAF - UNII:WS0Y3M85RF) BAY LEAF 4 [hp_X] in 20 mL MARSDENIA CONDURANGO BARK (UNII: R23QIR6YBA) (MARSDENIA CONDURANGO BARK - UNII:R23QIR6YBA) MARSDENIA CONDURANGO BARK 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1103-8 1 in 1 CARTON 06/01/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1103, 62106-1149)