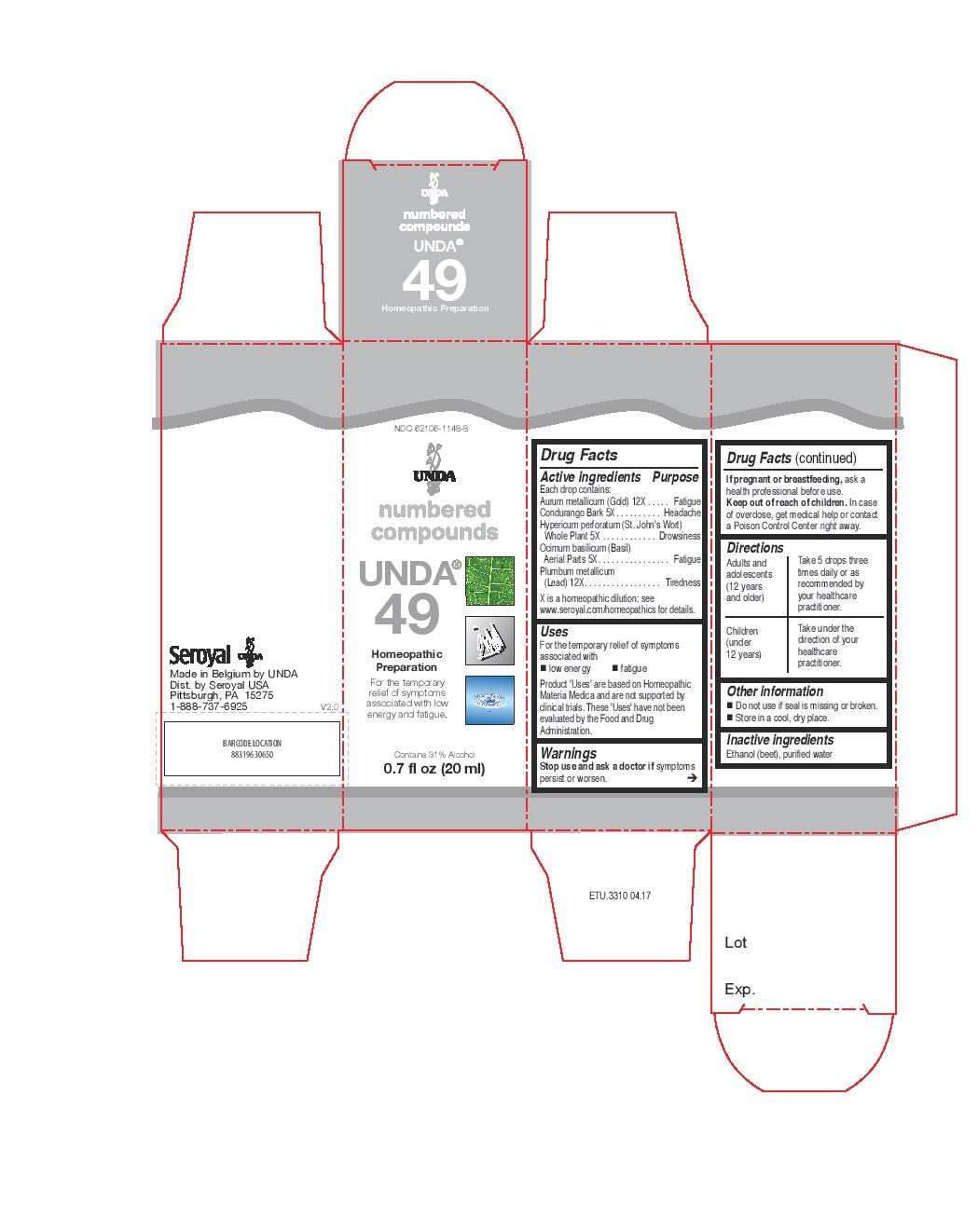
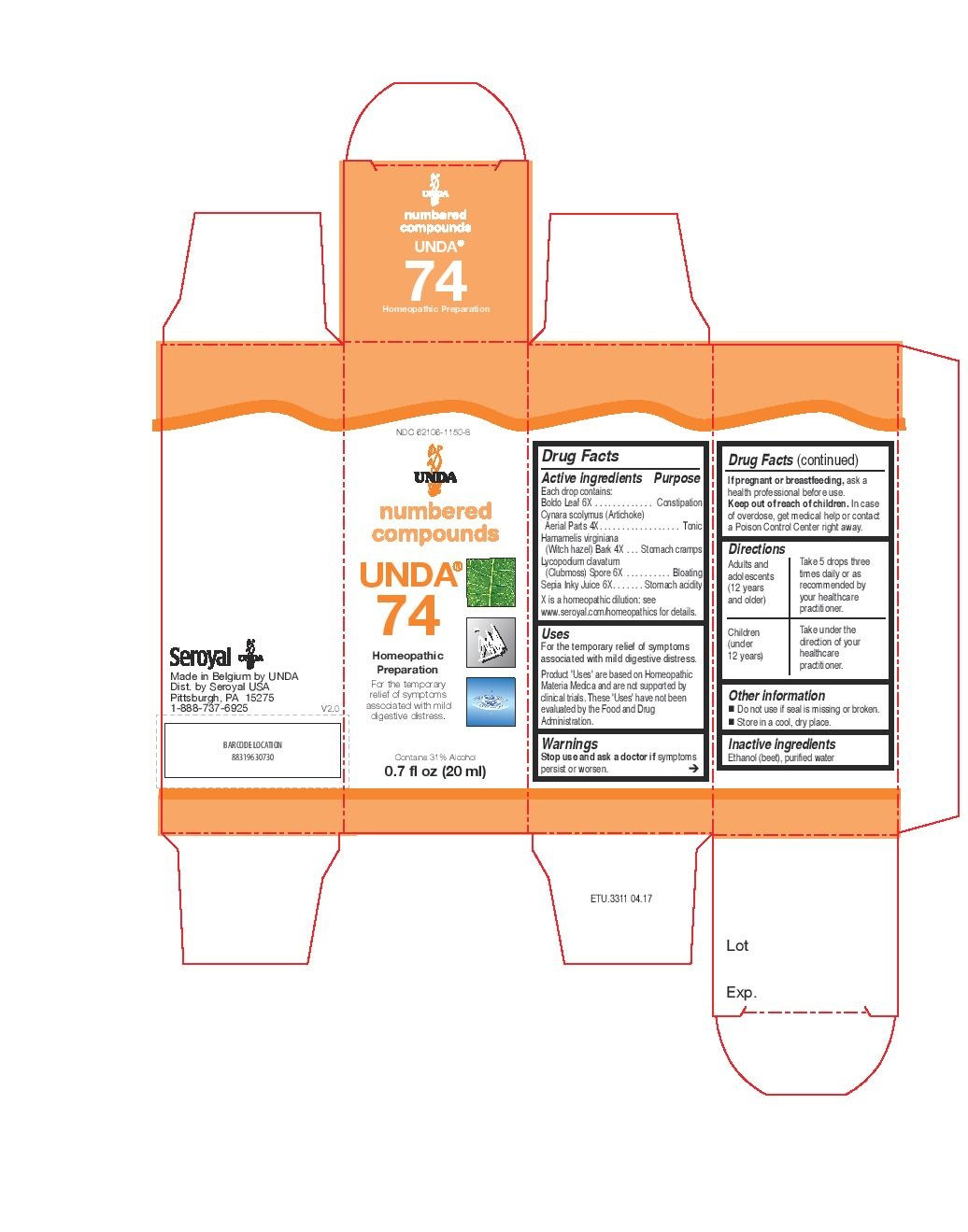
| NDC | 62106-1148-8, 62106-1150-8 |
| Set ID | 205ac635-cdf2-1b1d-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with low energy and fatigueDirections
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with mild digestive distress.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 49
aurum metallicum, condurango bark, hypericum perforatum, ocimum basilicum, plumbum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1148 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL MARSDENIA CONDURANGO BARK (UNII: R23QIR6YBA) (MARSDENIA CONDURANGO BARK - UNII:R23QIR6YBA) MARSDENIA CONDURANGO BARK 5 [hp_X] in 20 mL BASIL (UNII: 2U0KZP0FDW) (BASIL - UNII:2U0KZP0FDW) BASIL 5 [hp_X] in 20 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 12 [hp_X] in 20 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 5 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1148-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 UNDA 74
boldo leaf, cynara scolymus, hamamelis virginiana, lycopodium clavatum, sepia inky juice liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_X] in 20 mL CYNARA SCOLYMUS LEAF (UNII: B71UA545DE) (CYNARA SCOLYMUS LEAF - UNII:B71UA545DE) CYNARA SCOLYMUS LEAF 4 [hp_X] in 20 mL HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) (HAMAMELIS VIRGINIANA BARK - UNII:IH3063S9MY) HAMAMELIS VIRGINIANA BARK 4 [hp_X] in 20 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 6 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 6 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1150-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1148, 62106-1150)