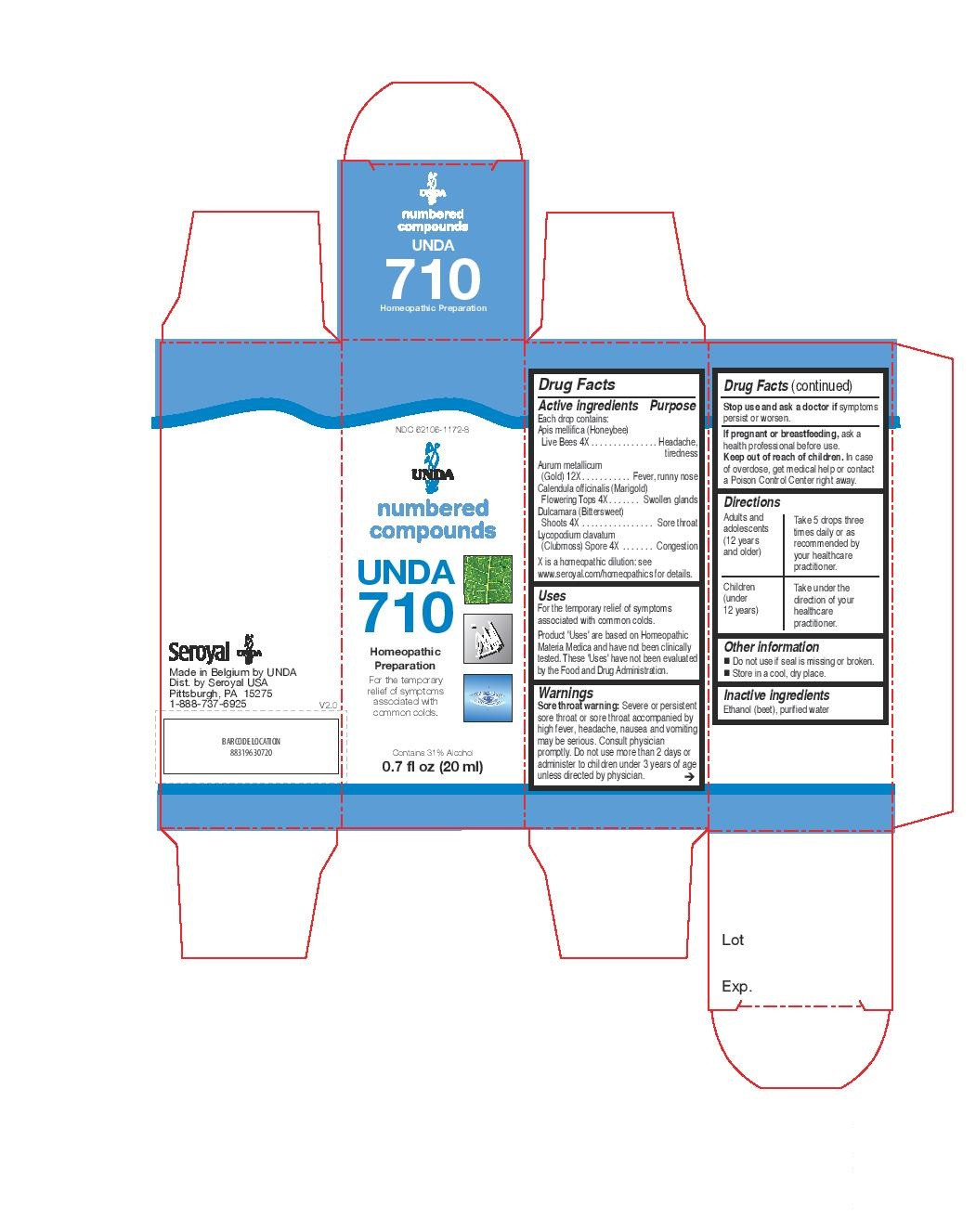
| NDC | 62106-1134-8, 62106-1172-8 |
| Set ID | 1f64baba-eb29-30c6-e054-00144ff8d46c |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you have
Persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
Cough that is accompanied by excessive phlegm (mucus) or fever.
Stop use and ask a doctor if
Cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or
persistent headache. A persistent cough may be a sign of a serious condition.
Symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warning
Sore throat warning: Severe or persistent sore throat or sore throat accompanied
by high fever, headache, nausea and vomiting maybe serious.
Consult physician promptly.
Do not use more than 2 days or administer to children under 3 years of age unless directed by physician.
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.In case of overdose, get medical help or contact a Poison Control Center right away.
- DO NOT USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 35
aurum metallicum, corallium rubrum exoskeleton, crataegus, grindelia, hamamelis virginiana, mentha piperita, myrtus communis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1134 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL CORALLIUM RUBRUM EXOSKELETON (UNII: 2CA71K0DLE) (CORALLIUM RUBRUM EXOSKELETON - UNII:2CA71K0DLE) CORALLIUM RUBRUM EXOSKELETON 12 [hp_X] in 20 mL CRATAEGUS FRUIT (UNII: Q21UUL2105) (CRATAEGUS FRUIT - UNII:Q21UUL2105) CRATAEGUS FRUIT 4 [hp_X] in 20 mL GRINDELIA HIRSUTULA FLOWERING TOP (UNII: IDB0NAZ6AI) (GRINDELIA HIRSUTULA FLOWERING TOP - UNII:IDB0NAZ6AI) GRINDELIA HIRSUTULA FLOWERING TOP 4 [hp_X] in 20 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 4 [hp_X] in 20 mL MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 4 [hp_X] in 20 mL MYRTUS COMMUNIS TOP (UNII: 367E55FXGW) (MYRTUS COMMUNIS TOP - UNII:367E55FXGW) MYRTUS COMMUNIS TOP 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1134-8 1 in 1 CARTON 09/10/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/10/2015 UNDA 710
apis mellifica, calendula officinalis, lycopodium clavatum, dulcamara, aurum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1172 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 20 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 4 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 4 [hp_X] in 20 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1172-8 1 in 1 CARTON 09/10/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/10/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1134, 62106-1172)