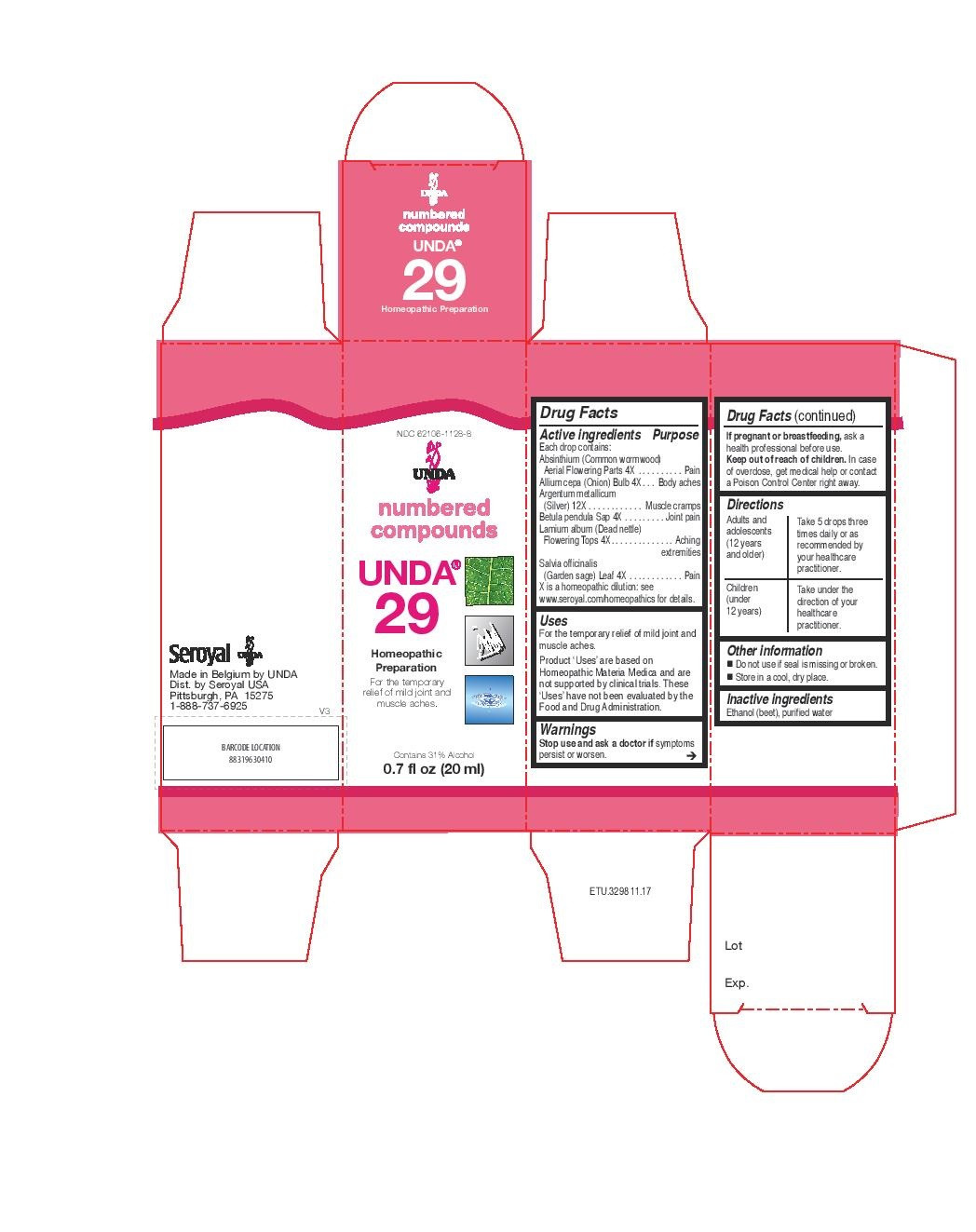
| NDC | 62106-1128-8, 62106-1137-8 |
| Set ID | 20484d33-4389-1a85-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the relief of symptoms associated with
minor difficulties in urination.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 29
absinthium, allium cepa, argentum metallicum, betula pendula sap, lamium album, salvia officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1128 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAMIUM ALBUM (UNII: 046Y1357I6) (LAMIUM ALBUM - UNII:046Y1357I6) LAMIUM ALBUM 4 [hp_X] in 20 mL WORMWOOD (UNII: F84709P2XV) (WORMWOOD - UNII:F84709P2XV) WORMWOOD 4 [hp_X] in 20 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL BETULA PENDULA WOOD (UNII: 568ZVX88G6) (BETULA PENDULA WOOD - UNII:568ZVX88G6) BETULA PENDULA WOOD 4 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1128-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 UNDA 38
aluminium metallicum, argentum metallicum, inula helenium, sarsaparilla, stigmata maidis, valeriana officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1137 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL INULA HELENIUM ROOT (UNII: E55SMD6DA8) (INULA HELENIUM ROOT - UNII:E55SMD6DA8) INULA HELENIUM ROOT 4 [hp_X] in 20 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 4 [hp_X] in 20 mL CORN SILK (UNII: 7D3VB244UX) (CORN SILK - UNII:7D3VB244UX) CORN SILK 4 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1137-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1128, 62106-1137)