| NDC | 62106-1127-8, 62106-1133-8 |
| Set ID | 182f4ad8-edf9-468c-e054-00144ff8d46c |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
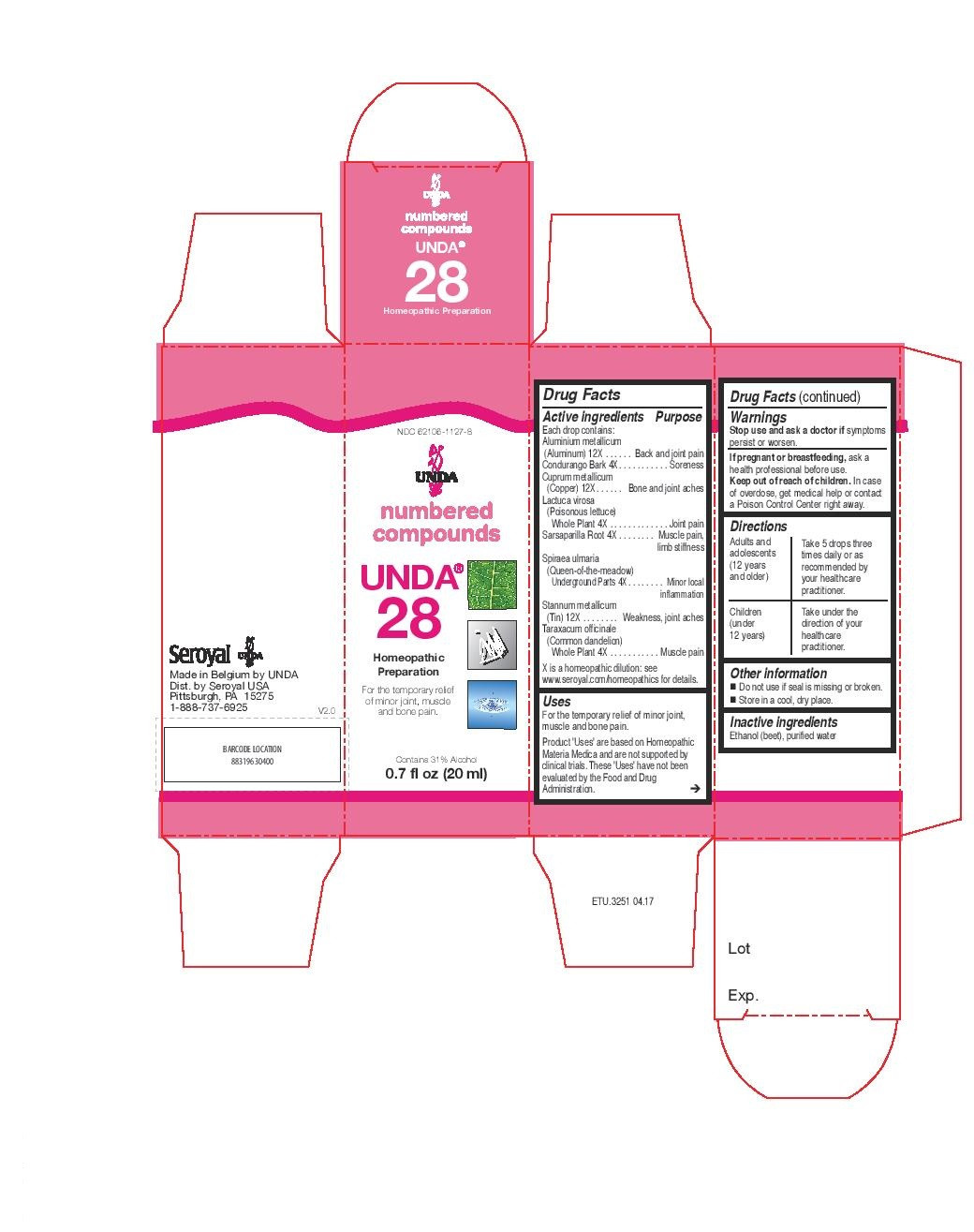
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Aluminium metallicum (Aluminum) 12X
Condurango Bark 4X
Cuprum metallicum (Copper) 12X
Lactuca virosa (Poisonous lettuce) Whole Plant 4X
Sarsaparilla Root 4X
Spiraea ulmaria (Queen-of-the-meadow) Underground Parts 4X
Stannum metallicum (Tin) 12X
Taraxacum officinale (Common dandelion) Whole Plant 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
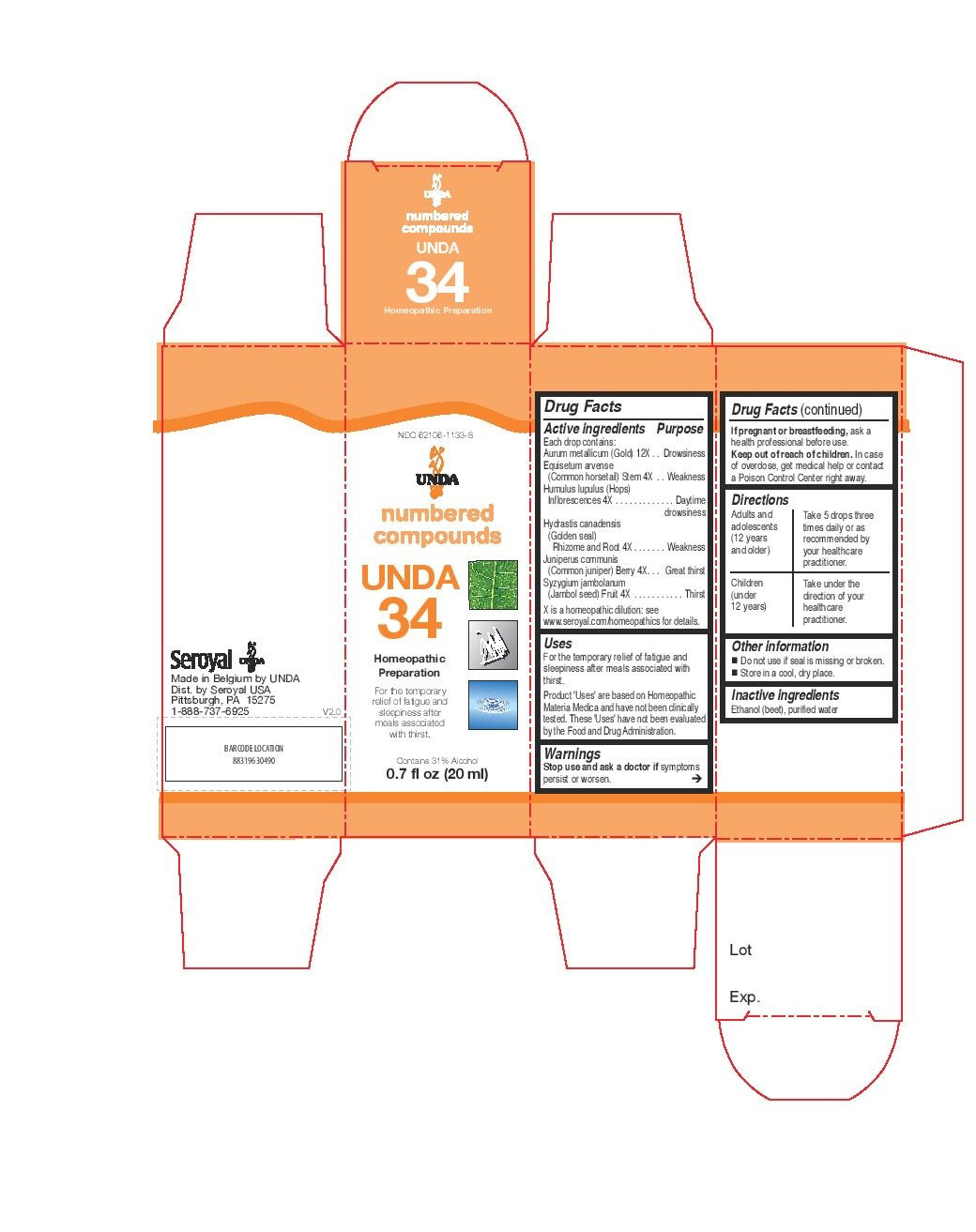
INDICATIONS & USAGE
Uses
For the temporary relief of fatigue and sleepiness after meals associated with thirst.Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 28
lactuca virosa, taraxacum officinale, sarsaparilla, spiraea ulmaria, condurango, aluminium metallicum, cuprum metallicum, stannum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 4 [hp_X] in 20 mL FILIPENDULA ULMARIA ROOT (UNII: 997724QNDS) (FILIPENDULA ULMARIA ROOT - UNII:997724QNDS) FILIPENDULA ULMARIA ROOT 4 [hp_X] in 20 mL ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 20 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 12 [hp_X] in 20 mL MARSDENIA CONDURANGO BARK (UNII: R23QIR6YBA) (MARSDENIA CONDURANGO BARK - UNII:R23QIR6YBA) MARSDENIA CONDURANGO BARK 4 [hp_X] in 20 mL TARAXACUM OFFICINALE WHOLE (UNII: 7C42E2D7B0) (TARAXACUM OFFICINALE WHOLE - UNII:7C42E2D7B0) TARAXACUM OFFICINALE WHOLE 4 [hp_X] in 20 mL LACTUCA VIROSA (UNII: 6D74QW4H67) (LACTUCA VIROSA - UNII:6D74QW4H67) LACTUCA VIROSA 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1127-8 1 in 1 CARTON 06/10/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/10/2015 UNDA 34
humulus lupulus, juniperus communis, equisetum arvense, hydrastis canadensis, syzygium jambolanum, aurum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOPS (UNII: 01G73H6H83) (HOPS - UNII:01G73H6H83) HOPS 4 [hp_X] in 20 mL JUNIPERUS COMMUNIS WHOLE (UNII: 464910T5N9) (JUNIPERUS COMMUNIS WHOLE - UNII:464910T5N9) JUNIPERUS COMMUNIS WHOLE 4 [hp_X] in 20 mL EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) (EQUISETUM ARVENSE BRANCH - UNII:1L0VKZ185E) EQUISETUM ARVENSE BRANCH 4 [hp_X] in 20 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 4 [hp_X] in 20 mL SYZYGIUM CUMINI SEED (UNII: 820LSF646I) (SYZYGIUM CUMINI SEED - UNII:820LSF646I) SYZYGIUM CUMINI SEED 4 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1133-8 1 in 1 CARTON 06/10/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/10/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1127, 62106-1133)