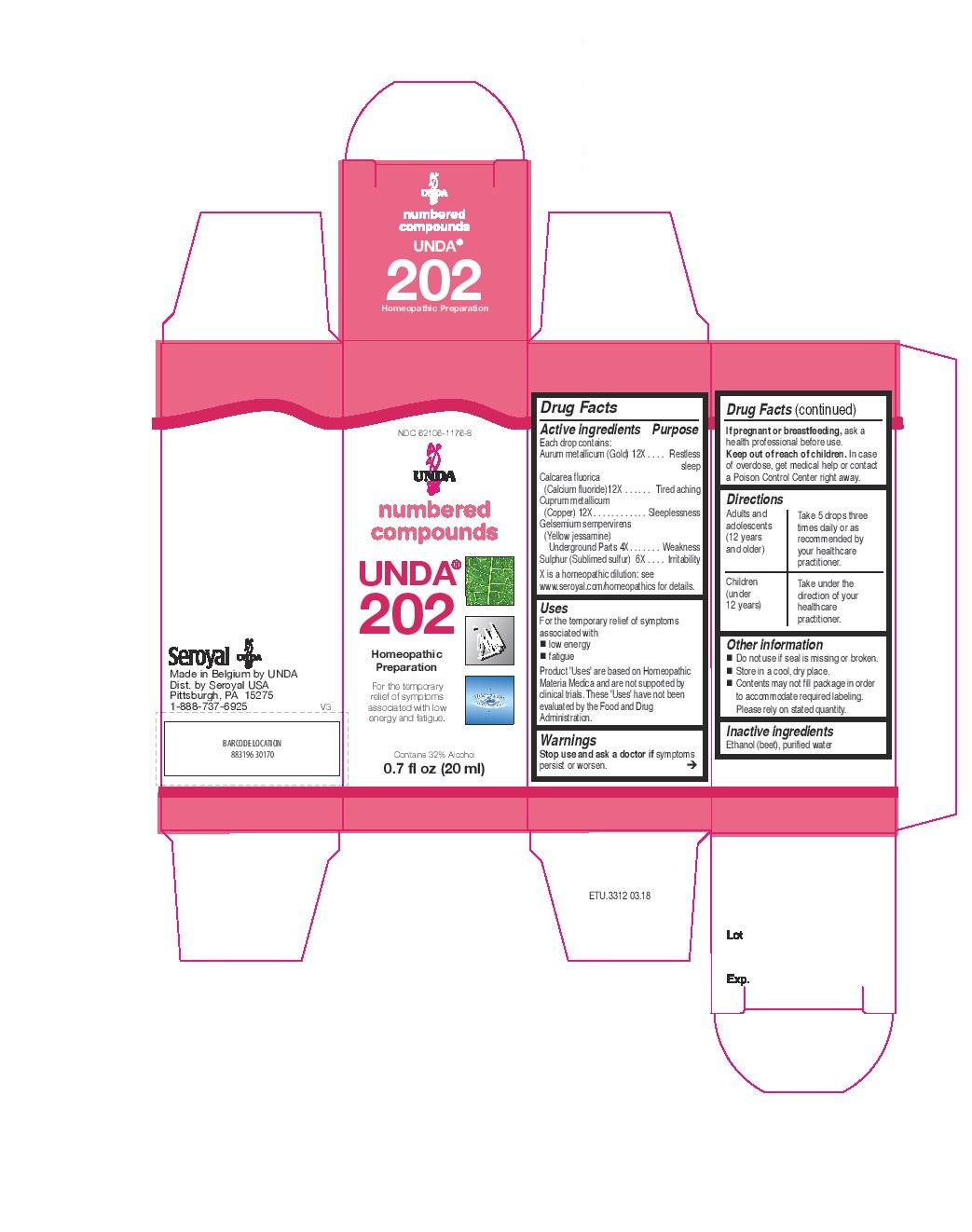
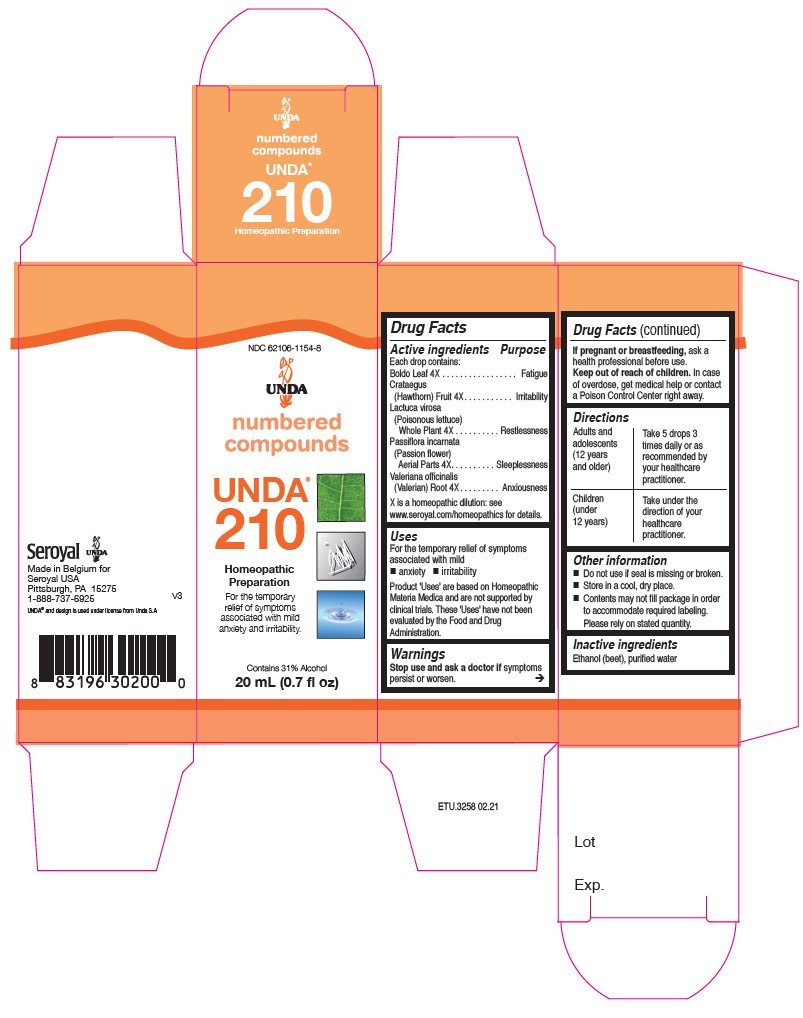
| NDC | 62106-1154-8, 62106-1176-8 |
| Set ID | 205b68ed-ff41-3559-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with mild anxiety and irritabilityDirections
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 210
boldo leaf, crataegus, lactuca virosa, passiflora incarnata, valeriana officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1154 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL CRATAEGUS FRUIT (UNII: Q21UUL2105) (CRATAEGUS FRUIT - UNII:Q21UUL2105) CRATAEGUS FRUIT 4 [hp_X] in 20 mL LACTUCA VIROSA (UNII: 6D74QW4H67) (LACTUCA VIROSA - UNII:6D74QW4H67) LACTUCA VIROSA 4 [hp_X] in 20 mL PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 4 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1154-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 UNDA 202
aurum metallicum, calcarea fluorica, cuprum metallicum, gelsemium sempervirens, sulphur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1176 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] in 20 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 20 mL GELSEMIUM SEMPERVIRENS WHOLE (UNII: R0519OZO3K) (GELSEMIUM SEMPERVIRENS WHOLE - UNII:R0519OZO3K) GELSEMIUM SEMPERVIRENS WHOLE 4 [hp_X] in 20 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1176-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1176, 62106-1154)