| NDC | 62106-1102-8, 62106-1106-8, 62106-1115-8, 62106-1144-8, 62106-1147-8 |
| Set ID | 1583cf73-c22c-0fa8-e054-00144ff8d46c |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- WARNINGS
- PURPOSE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
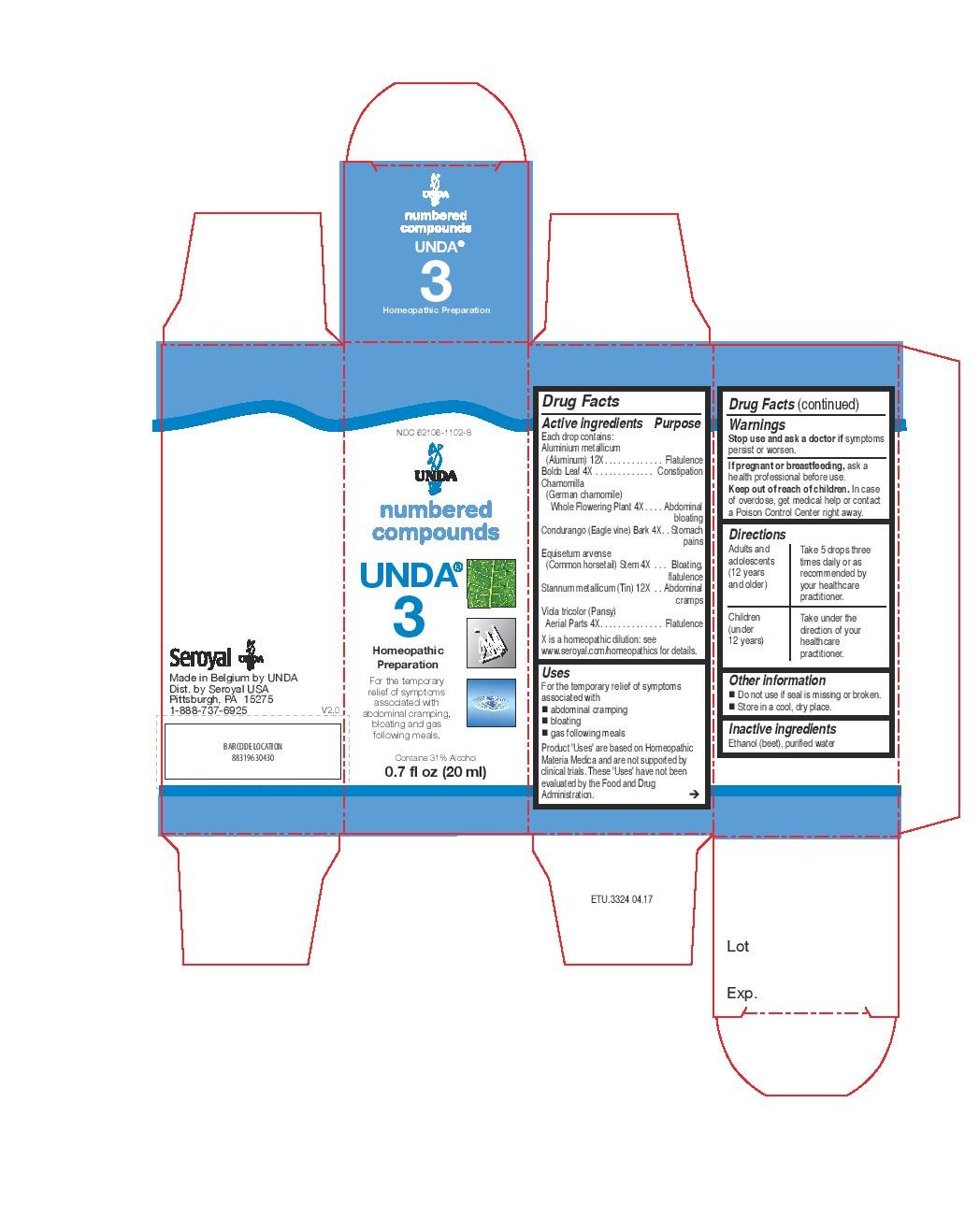
Uses
For the temporary relief of symptoms associated with abdominal cramping, bloating and gas following meals.Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
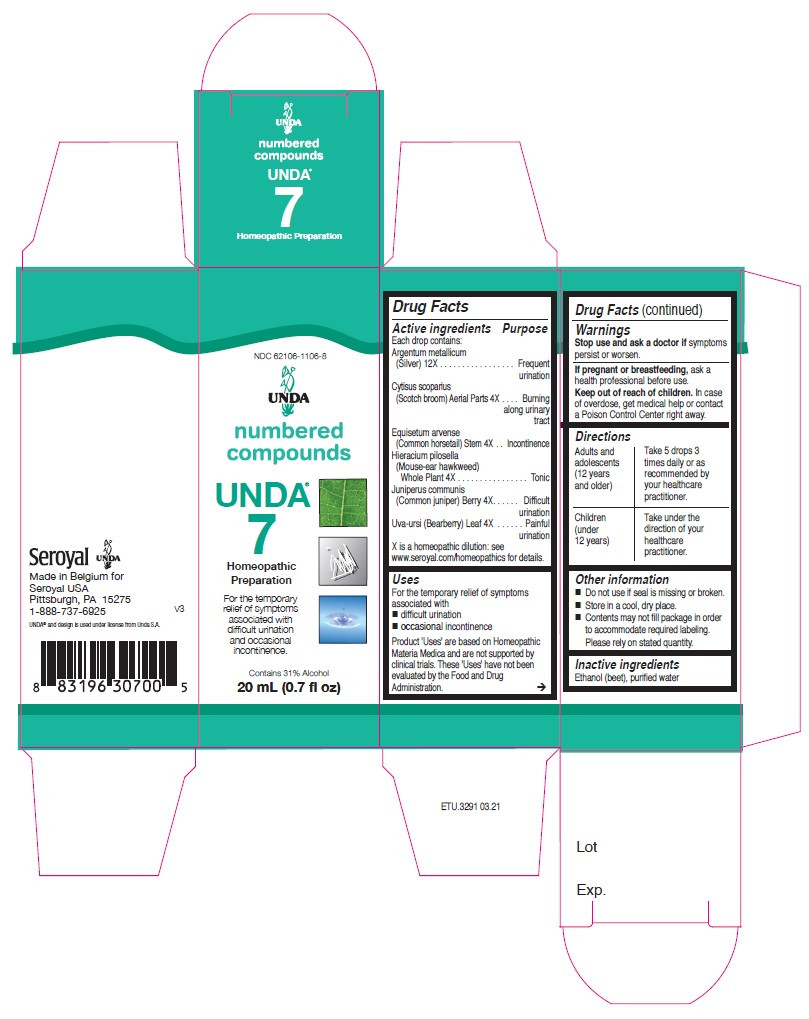
For the temporary relief of symptoms
associated with difficult urination
and occasional incontinence.Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
-
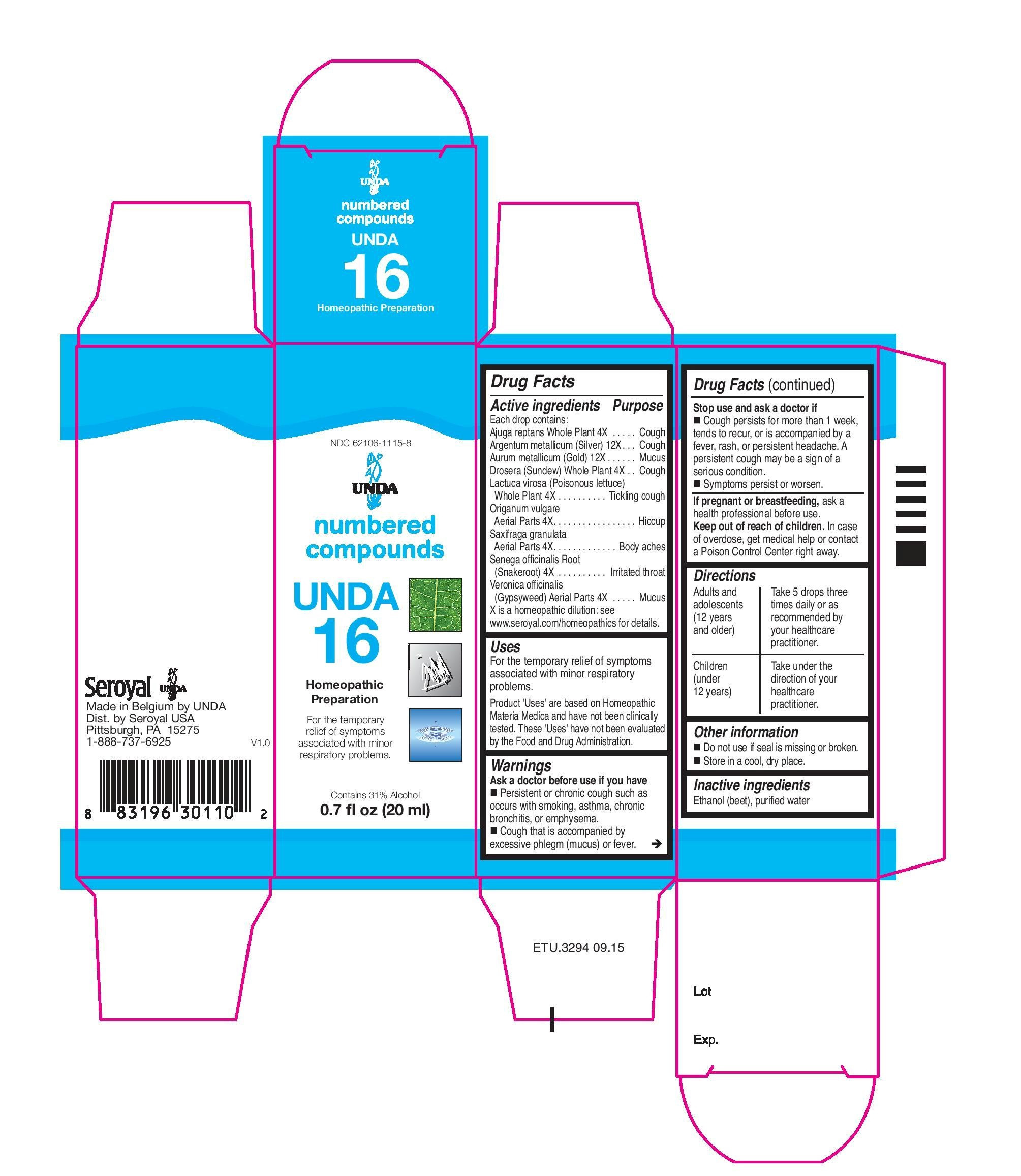
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Saxifraga granulata Aerial Parts. 4X
Origanum vulgare Aerial Parts. 4X
Lactuca virosa (Poisonous lettuce) Whole Plant . 4X
Veronica officinalis (Gypsyweed) Aerial Parts . 4X
Ajuga reptansWhole Plant. 4X
Drosera (Sundew)Whole Plant . 4X
Senega officinalis Root . 4X
Aurum metallicum (Gold) . 12X
Argentum metallicum (Silver) . 12X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Indications
To help with calcium absorption and for symptoms associated
with minor respiratory problems.Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
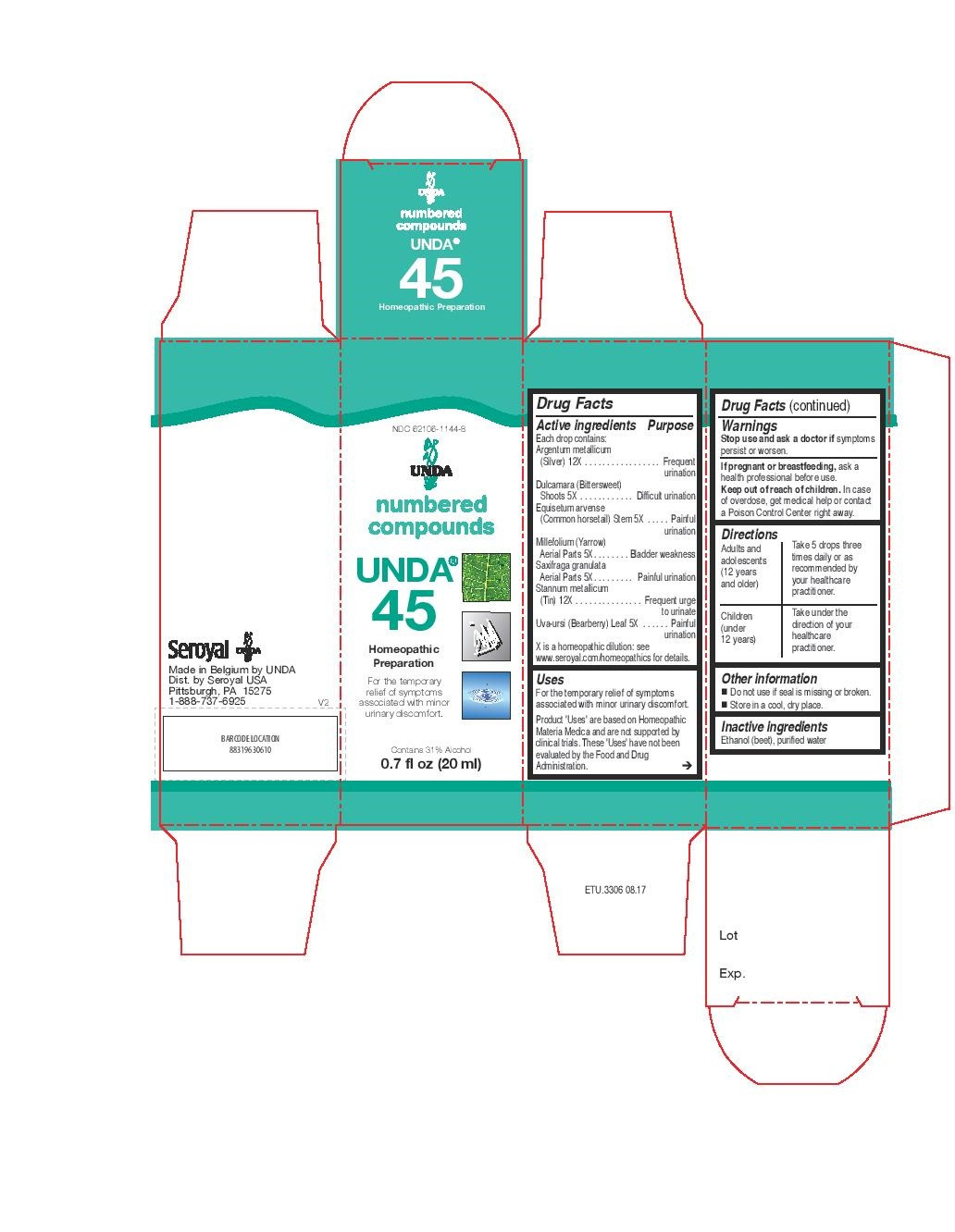
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with minor urinary discomfort.Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Arsenicum album (Arsenic trioxide) 12X
Aurum metallicum (Gold) 12X
Calendula officinalis (Marigold) Aerial Parts 4X
Condurango Bark 4X
Lycopodium clavatum (Clubmoss) Spore 4X
Plumbum metallicum (Lead) 12X
Sabina (Savin) Twig 4X
Thuja occidentalis (White cedar) Leafy Twigs 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
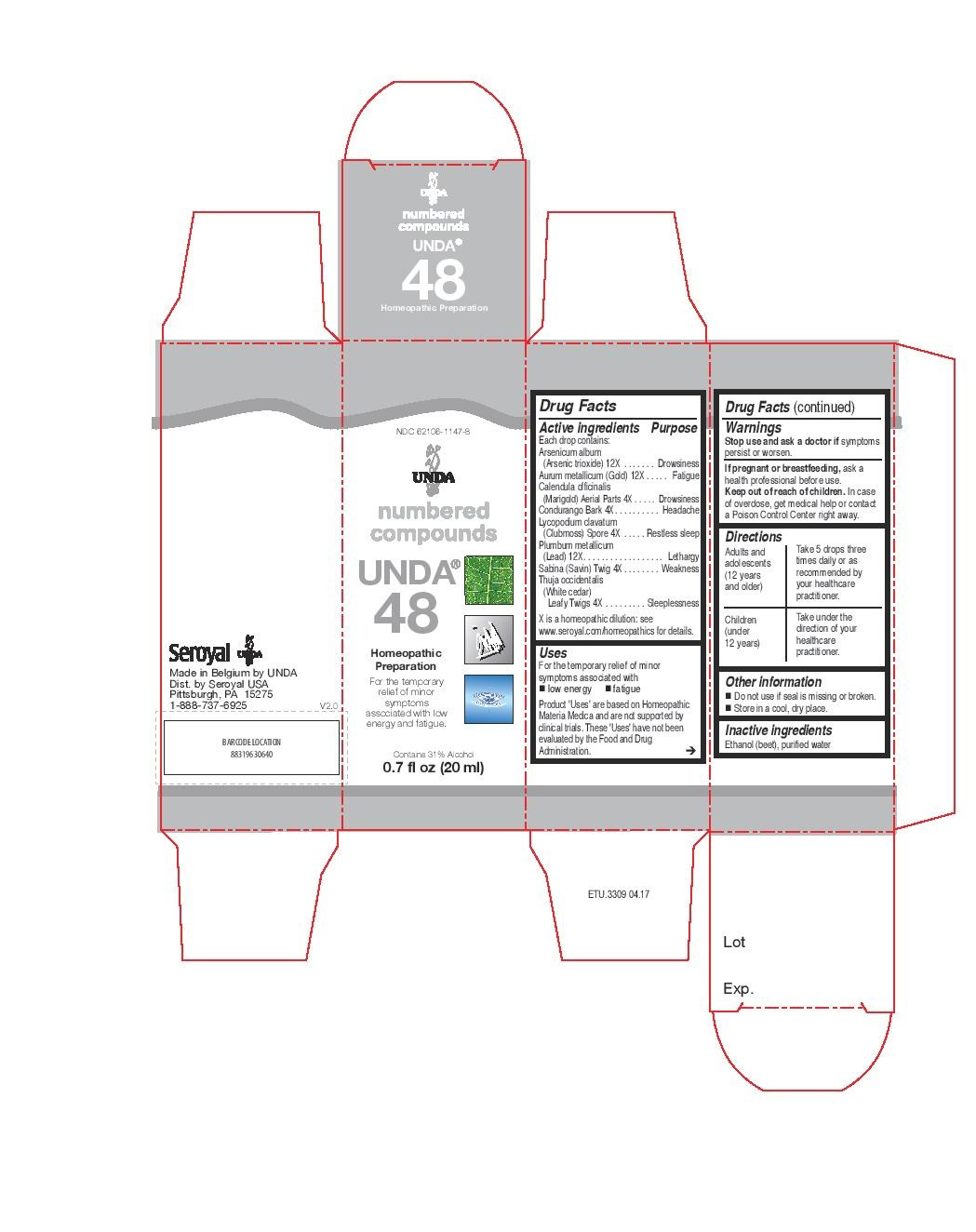
INDICATIONS & USAGE
Uses
For the temporary relief of minor symptoms associated with low energy and fatigue.Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 16
saxifraga granulata, origanum vulgare, lactuca virosa, veronica officinalis, ajuga reptans, drosera, senega officinalis, aurum metallicum, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERONICA OFFICINALIS FLOWERING TOP (UNII: 9IH82J936J) (VERONICA OFFICINALIS FLOWERING TOP - UNII:9IH82J936J) VERONICA OFFICINALIS FLOWERING TOP 4 [hp_X] in 20 mL AJUGA REPTANS WHOLE (UNII: E678D1QH0K) (AJUGA REPTANS WHOLE - UNII:E678D1QH0K) AJUGA REPTANS WHOLE 4 [hp_X] in 20 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 4 [hp_X] in 20 mL POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 4 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL SAXIFRAGA GRANULATA WHOLE (UNII: W1LUS9R48G) (SAXIFRAGA GRANULATA WHOLE - UNII:W1LUS9R48G) SAXIFRAGA GRANULATA WHOLE 4 [hp_X] in 20 mL ORIGANUM VULGARE SUBSP. HIRTUM WHOLE (UNII: 38SNL0F81Z) (ORIGANUM VULGARE SUBSP. HIRTUM WHOLE - UNII:38SNL0F81Z) ORIGANUM VULGARE SUBSP. HIRTUM WHOLE 4 [hp_X] in 20 mL LACTUCA VIROSA (UNII: 6D74QW4H67) (LACTUCA VIROSA - UNII:6D74QW4H67) LACTUCA VIROSA 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1115-8 1 in 1 CARTON 05/07/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/07/2015 UNDA 3
equisetum arvense stem, chamomilla, condurango bark, viola tricolor, boldo leaf, aluminium metallicum, stannum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) (EQUISETUM ARVENSE TOP - UNII:1DP6Y6B65Z) EQUISETUM ARVENSE TOP 4 [hp_X] in 20 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 4 [hp_X] in 20 mL MARSDENIA CONDURANGO BARK (UNII: R23QIR6YBA) (MARSDENIA CONDURANGO BARK - UNII:R23QIR6YBA) MARSDENIA CONDURANGO BARK 4 [hp_X] in 20 mL VIOLA TRICOLOR (UNII: 9Q24RAI43V) (VIOLA TRICOLOR - UNII:9Q24RAI43V) VIOLA TRICOLOR 4 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1102-8 1 in 1 CARTON 05/07/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/07/2015 UNDA 48
condurango, calendula officinalis, lycopodium clavatum, sabina, thuja occidentalis, aurum metallicum, plumbum metallicum, arsenicum album liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1147 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MARSDENIA CONDURANGO BARK (UNII: R23QIR6YBA) (MARSDENIA CONDURANGO BARK - UNII:R23QIR6YBA) MARSDENIA CONDURANGO BARK 4 [hp_X] in 20 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 4 [hp_X] in 20 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 20 mL JUNIPERUS SABINA LEAF (UNII: 0R715588D2) (JUNIPERUS SABINA LEAF - UNII:0R715588D2) JUNIPERUS SABINA LEAF 4 [hp_X] in 20 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 4 [hp_X] in 20 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 12 [hp_X] in 20 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1147-8 1 in 1 CARTON 05/08/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/08/2015 UNDA 7
equisetum arvense, uva-ursi, juniperus communis, cytisus scoparius, hieraciumpilosella, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HIERACIUM PILOSELLA WHOLE (UNII: 74068FOS7R) (HIERACIUM PILOSELLA WHOLE - UNII:74068FOS7R) HIERACIUM PILOSELLA WHOLE 4 [hp_X] in 20 mL EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) (EQUISETUM ARVENSE TOP - UNII:1DP6Y6B65Z) EQUISETUM ARVENSE TOP 4 [hp_X] in 20 mL ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) (ARCTOSTAPHYLOS UVA-URSI LEAF - UNII:3M5V3D1X36) ARCTOSTAPHYLOS UVA-URSI LEAF 4 [hp_X] in 20 mL JUNIPERUS COMMUNIS WHOLE (UNII: 464910T5N9) (JUNIPERUS COMMUNIS WHOLE - UNII:464910T5N9) JUNIPERUS COMMUNIS WHOLE 4 [hp_X] in 20 mL CYTISUS SCOPARIUS FLOWERING TOP (UNII: XZC6H8R666) (CYTISUS SCOPARIUS FLOWERING TOP - UNII:XZC6H8R666) CYTISUS SCOPARIUS FLOWERING TOP 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1106-8 1 in 1 CARTON 05/07/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/07/2015 UNDA 45
equisetum arvense, millefolium, saxifraga granulata, dulcamara, uva-ursi, argentum metallicum, stannum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SAXIFRAGA GRANULATA WHOLE (UNII: W1LUS9R48G) (SAXIFRAGA GRANULATA WHOLE - UNII:W1LUS9R48G) SAXIFRAGA GRANULATA WHOLE 5 [hp_X] in 20 mL EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) (EQUISETUM ARVENSE TOP - UNII:1DP6Y6B65Z) EQUISETUM ARVENSE TOP 5 [hp_X] in 20 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 5 [hp_X] in 20 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 5 [hp_X] in 20 mL ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) (ARCTOSTAPHYLOS UVA-URSI LEAF - UNII:3M5V3D1X36) ARCTOSTAPHYLOS UVA-URSI LEAF 5 [hp_X] in 20 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1144-8 1 in 1 CARTON 05/07/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/07/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1102, 62106-1106, 62106-1115, 62106-1144, 62106-1147)