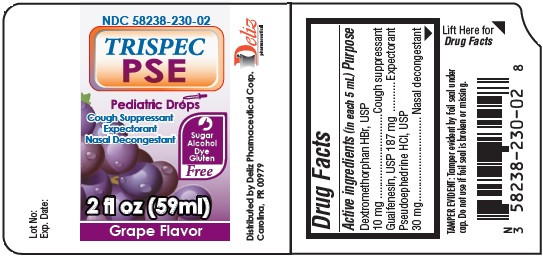
| NDC | 58238-230-02 |
| Set ID | b4630613-c78c-4f97-bd6c-0476899f5b90 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Deliz Pharmaceutical Corp |
| Generic Name | |
| Product Class | alpha-Adrenergic Agonist |
| Product Number | |
| Application Number | PART341 |
- Drug Facts
- Active ingredients (in each 5 mL)
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by excessive phlegm (mugus)
Stop use and ask a doctor if
- cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
- symptoms do not improve within 7 days or are acompanied by fever
- nervousness, dizziness, or sleeplessness occur
- Directions
- Other information
- Inactive ingredients
- Questions?
- TRISPEC PSE Pediatric Drops Cough Suppressant Expectorant Nasal Decongestant GRAPE Flavor 2oz/59ml (58238-230-02)
-
INGREDIENTS AND APPEARANCE
TRISPEC PSE PEDIATRIC DROPS COUGH SUPPRESSANT EXPECTORANT NASAL DECONGESTANT GRAPE FLAVOR
dextromethorphan hydrobromide, guaifenesin, pseudoephedrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58238-230 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 187 mg in 5 mL PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor GRAPE (Grape Flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58238-230-02 1 in 1 CARTON 02/27/2014 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/27/2014 Labeler - Deliz Pharmaceutical Corp (826391138) Establishment Name Address ID/FEI Business Operations Woodfield Pharmaceutical, LLC 079398730 manufacture(58238-230)