| NDC | 13811-007-10 |
| Set ID | f05f4cec-3932-4198-9c86-d03bd13bc69d |
| Category | DIETARY SUPPLEMENT |
| Packager | Trigen Laboratories, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
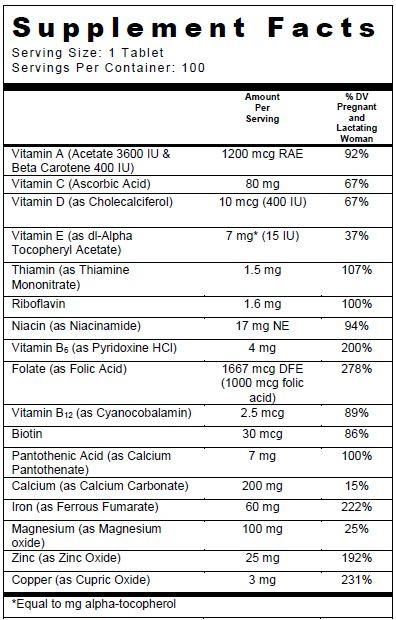
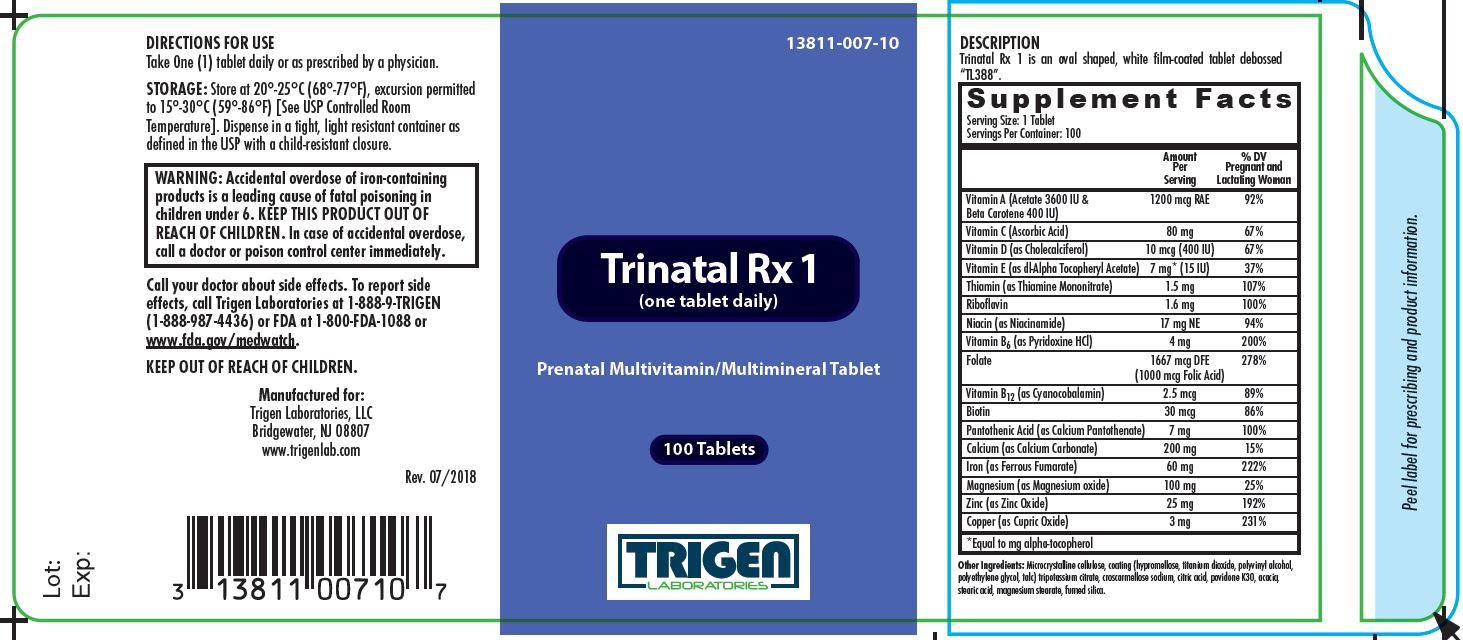
SUPPLEMENT FACTS

Other Ingredients: Microcrystalline cellulose, coating (hypromellose, titanium dioxide, polyvinyl alcohol, polyethylene glycol, talc) tripotassium citrate, croscarmellose sodium, citric acid, povidone K30, acacia, stearic acid, magnesium stearate, fumed silica.
Trinatal Rx 1 tablets help assure an adequate intake of the vitamins and minerals listed. Folate supplementation may be recommended for individuals who have higher-than-normal demands for folate such as pregnant women.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive. Pernicious anemia should be excluded before using this product since folic acid may mask the symptoms of pernicious anemia. The calcium content should be considered before prescribing for patients with history of kidney stones. Do not exceed the recommended dose.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone test, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Safety and effectiveness in elderly patients have not been established.
- DRUG INTERACTIONS
-
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parenteral administration of folic acid. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-888-9-TRIGEN (1-888-987-4436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Customer Service: 1-888-987-4436 Rev. 07/2018
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807

- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
TRINATAL RX 1
acetate ion, .beta.-carotene, cholecalciferol, .alpha.-tocopherol acetate, dl-, ascorbic acid, folic acid, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, magnesium oxide, ferrous fumarate, cupric oxide, and zinc oxide tablet, film coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength acetate ion (UNII: 569DQM74SC) (acetate ion - UNII:569DQM74SC) acetate ion 3600 [iU] .beta.-carotene (UNII: 01YAE03M7J) (.beta.-carotene - UNII:01YAE03M7J) .beta.-carotene 400 [iU] cholecalciferol (UNII: 1C6V77QF41) (cholecalciferol - UNII:1C6V77QF41) cholecalciferol 400 [iU] .alpha.-tocopherol acetate, dl- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 15 [iU] ascorbic acid (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ascorbic acid 80 mg folic acid (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) folic acid 1 mg thiamine mononitrate (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.5 mg riboflavin (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) riboflavin 1.6 mg niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) niacinamide 17 mg pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) pyridoxine Hydrochloride 4 mg cyanocobalamin (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) cyanocobalamin 2.5 ug biotin (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) biotin 30 ug calcium pantothenate (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 7 mg calcium carbonate (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) calcium carbonate 200 mg magnesium oxide (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) magnesium oxide 100 mg ferrous fumarate (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 60 mg cupric oxide (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 3 mg zinc oxide (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) zinc oxide 25 mg Inactive Ingredients Ingredient Name Strength cellulose, microcrystalline (UNII: OP1R32D61U) hypromelloses (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) talc (UNII: 7SEV7J4R1U) potassium citrate anhydrous (UNII: 86R1NVR0HW) croscarmellose sodium (UNII: M28OL1HH48) citric acid monohydrate (UNII: 2968PHW8QP) povidone K30 (UNII: U725QWY32X) acacia (UNII: 5C5403N26O) stearic acid (UNII: 4ELV7Z65AP) magnesium stearate (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-007-10 100 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 03/01/2009 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 19 mm Labeler - Trigen Laboratories, LLC (830479668)