| NDC | 13811-536-30 |
| Set ID | 371e914f-e65a-4ad5-aeb6-54d8c7ab6838 |
| Category | DIETARY SUPPLEMENT |
| Packager | Trigen Laboratories, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
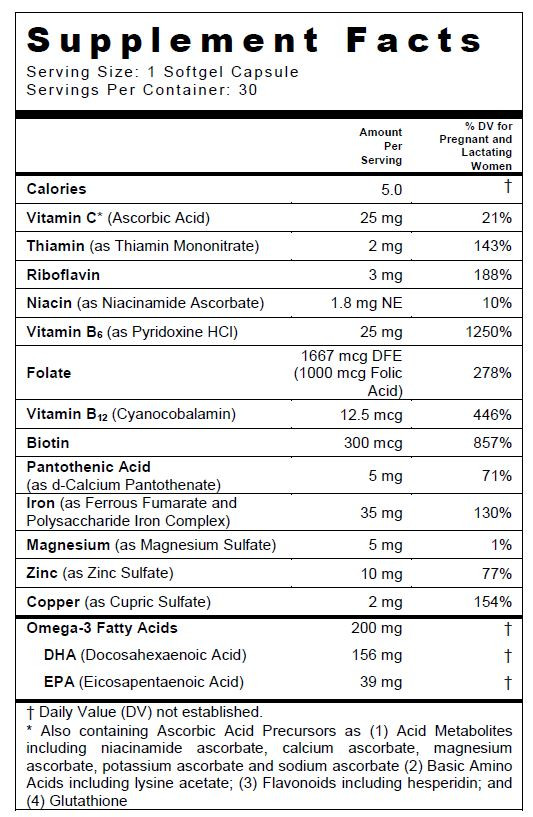
SUPPLEMENT FACTS

Other Ingredients: Bovine Gelatin, Glycerin, Yellow Beeswax, Soy Lecithin, Purified Water, Orange Cream Flavor O.S, Sorbitol, Titanium Dioxide, Ethyl Vanillin, FD&C Blue #1.
THIS PRODUCT CONTAINS SOY AND FISH (TUNA, MAY INCLUDE ANCHOVY AND/OR SARDINE).
Taron™-C DHA is a prescription multivitamin/mineral indicated to provide omega-3 fatty acid supplementation throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. Taron™-C DHA may be useful in improving the nutritional status of women prior to conception. - CONTRAINDICATIONS
-
WARNINGS
Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
WARNING:Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. -
PRECAUTIONS
Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric populations have not been established.
Geriatric Use: Safety and effectiveness in elderly populations have not been established.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin. - DRUG INTERACTIONS
-
ADVERSE REACTIONS
Folic Acid: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, giving Taron™-C DHA after meals may control occasional G.I. disturbances. Taron™-C DHA is best absorbed when taken at bedtime. -
OVERDOSAGE
Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness, and coma. The estimated overdose of orally ingested iron is 300-mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Taron™-C DHA should be stored beyond the reach of children to prevent against accidental iron poisoning.
Treatment: For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk. Administer intravenous fluids and electrolytes and use oxygen. - DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-888-9-TRIGEN (1-888-987-4436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.Rev. 08/2018
Customer Service: 1-888-987-4436
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807
www.trigenlab.com
- PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
TARON-C DHA
ferrous fumarate, iron, ascorbic acid, folic acid, thiamine mononitrate, riboflavin, niacin, calcium pantothenate, pyridoxine hydrochloride, biotin, cyanocobalamin, cupric sulfate, magnesium sulfate, zinc sulfate, and omega-3 fatty acids capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-536 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ferrous fumarate (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 17.5 mg IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 17.5 mg ascorbic acid (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ascorbic acid 25 mg folic acid (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) folic acid 1 mg thiamine mononitrate (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 2 mg riboflavin (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) riboflavin 3 mg Niacin (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) Niacin 1.8 mg calcium pantothenate (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 5 mg pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) pyridoxine Hydrochloride 25 mg biotin (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) biotin 300 ug cyanocobalamin (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) cyanocobalamin 12.5 ug Cupric Sulfate (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE ANHYDROUS 5 mg zinc sulfate (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10 mg Omega-3 fatty acids (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) Omega-3 fatty acids 200 mg Inactive Ingredients Ingredient Name Strength GELATIN TYPE B BOVINE (160 BLOOM) (UNII: 1T8387508X) glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) lecithin, soybean (UNII: 1DI56QDM62) yellow wax (UNII: 2ZA36H0S2V) titanium dioxide (UNII: 15FIX9V2JP) ethyl vanillin (UNII: YC9ST449YJ) FD&C blue No. 1 (UNII: H3R47K3TBD) SORBITOL (UNII: 506T60A25R) ORANGE OIL (UNII: AKN3KSD11B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-536-30 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 12/01/2009 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor imprint scoring 1 shape size (solid drugs) 15 mm Labeler - Trigen Laboratories, LLC (830479668)