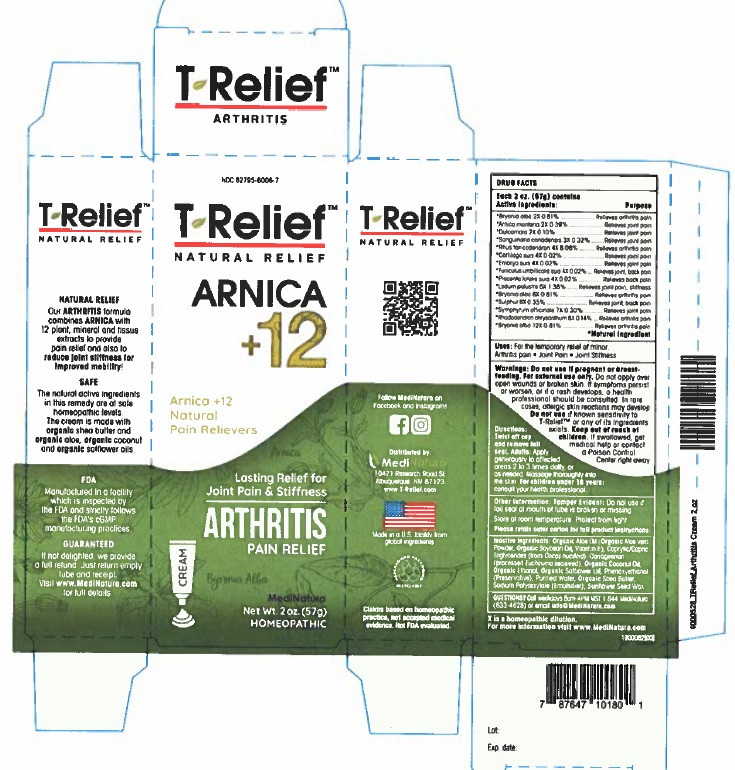
| NDC | 62795-6006-7 |
| Set ID | 861754ec-d9c0-45e3-9058-7e44dadcba64 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | MediNatura Inc |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
Active Ingredients
Each 50g contains
Active ingredients Purpose
*Arnica montana 2X 0.39%...............Relieves joint pain
*Bryonia alba 2X 0.81%.............................Relieves arthritis pain
*Bryonia alba 6X 0.81%.............................Relieves arthritis pain
*Bryonia alba 12X 0.81%...........................Relieves arthritis pain
*Cartilago suis 4X 0.02%............................Relieves joint pain
*Dulcamara 2X 0.10%................................Relieves joint pain
*Embryo suis 4X 0.02%.............................Relieves joint pain
*Funiculus umbilicalis suis 4X 0.02%............Relieves joint, back pain
*Ledum palustre 6X 1.38%.........................Relieves joint pain, stiffness
*Placenta totalis suis 4X 0.02%...................Relieves back pain
*Rhododendron chrysanthum 8X 0.14%......Relieves arthritis pain
*Rhus toxicodendron 4X 8.08%...................Relieves arthritis pain
*Sanguinaria canadensis 3X 0.32%...............Relieves joint pain
*Sulphur 6X 0.35%. ....................................Relieves joint back, pain
*Symphytum officinale 7X 0.30%.................Relieves joint pain
*Natural Ingredients
- Uses
-
WARNINGS
Do not use if pregnant or breast-feeding. For external use only.Do not apply over open wounds or broken skin. If symptoms persist or worsen, or if a rash develops, a health professional should be consulted. In rare cases, allergic skin reactions may develop. Do not use if known sensitivity to T-ReliefTM Arthritis or any of its ingredients exists. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- DIRECTIONS
-
INACTIVE INGREDIENTS
Organic Aloe Oil (Organic Aloe vera Powder, Organic Soybean Oil, Vitamin E), Caprylic/Capric Triglycerides (from Cocos nucifera), Carrageenan (processed Eucheuma seaweed), Organic Coconut Oil, Ethanol (from Organic Cane), Organic Safflower Oil, Phenoxyethanol (Preservative), Purified Water, Organic Shea Butter, Sodium Polyacrylate (Emulsifier), Natural Sunflower Seed Wax
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
T-RELIEF ARTHRITIS
arnica montana root, bryonia alba whole, sus scrofa umbilical cord, solanum dulcamara top, sus scrofa embryo, sus scrofa umbilical cord, ledum palustre twig, sus scrofa placenta, rhododendron aureum leaf, toxicodendron pubescens leaf, sanguinaria canadensis root, sulfur, and comfrey root creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62795-6006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 2 [hp_X] in 1 g BRYONIA ALBA WHOLE (UNII: 56K0VVT47P) (BRYONIA ALBA WHOLE - UNII:56K0VVT47P) BRYONIA ALBA WHOLE 2 [hp_X] in 1 g SUS SCROFA UMBILICAL CORD (UNII: 118OYG6W3H) (SUS SCROFA UMBILICAL CORD - UNII:118OYG6W3H) SUS SCROFA UMBILICAL CORD 4 [hp_X] in 1 g SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 2 [hp_X] in 1 g SUS SCROFA EMBRYO (UNII: 9928MC12VO) (SUS SCROFA EMBRYO - UNII:9928MC12VO) SUS SCROFA EMBRYO 4 [hp_X] in 1 g SUS SCROFA CARTILAGE (UNII: 73ECW5WG2F) (SUS SCROFA CARTILAGE - UNII:73ECW5WG2F) SUS SCROFA CARTILAGE 4 [hp_X] in 1 g LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 6 [hp_X] in 1 g SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 4 [hp_X] in 1 g RHODODENDRON AUREUM LEAF (UNII: IV92NQJ73U) (RHODODENDRON AUREUM LEAF - UNII:IV92NQJ73U) RHODODENDRON AUREUM LEAF 8 [hp_X] in 1 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 4 [hp_X] in 1 g SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 3 [hp_X] in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] in 1 g COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 7 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) SOYBEAN OIL (UNII: 241ATL177A) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) CARRAGEENAN (UNII: 5C69YCD2YJ) COCONUT OIL (UNII: Q9L0O73W7L) SAFFLOWER OIL (UNII: 65UEH262IS) ISOHEXADECANE (UNII: 918X1OUF1E) PEG-8 DIMETHICONE (UNII: GIA7T764OD) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SHEA BUTTER (UNII: K49155WL9Y) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62795-6006-7 1 in 1 CARTON 01/31/2017 1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/31/2017 Labeler - MediNatura Inc (079324099) Establishment Name Address ID/FEI Business Operations MediNatura Inc 102783016 manufacture(62795-6006)