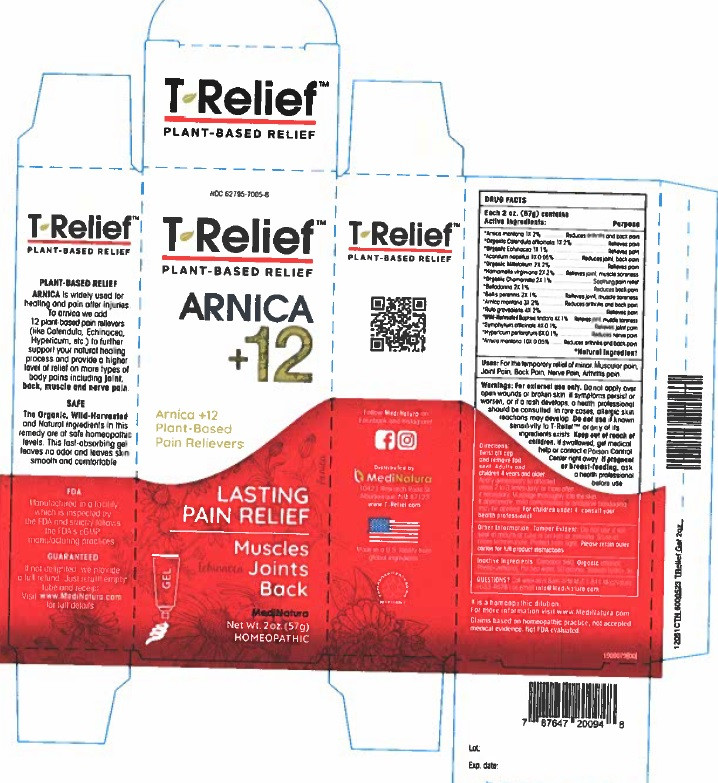
| NDC | 62795-7005-5, 62795-7005-6 |
| Set ID | edb2fe62-e990-4cef-8600-9d1b85bffa5a |
| Category | HUMAN OTC DRUG LABEL |
| Packager | MediNatura Inc |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS
Active ingredients Purpose
*Aconitum napellus 1X 0.5%................Reduces joint and back pain
*Arnica montana 1X 2%........Reduces joint and back pain
*Arnica montana 3X 2%..........Reduces joint and back pain
*Arnica montana10X 2%........Reduces joint and back pain
*Baptisia tinctoria 4X 1% ..................Reduces joint and muscle pain
*Belladonna 2X 1%...........................Reduces back pain
*Bellis perennis 2X 1%......................Relieves joint and muscle soreness
*Calendula officinalis 1X 1%..............Relieves pain
*Chamomilla 2X 1%.........................Soothing pain relief
*Echinacea 1X 1%............................Relieves pain
*Hamamelis virginiana 2X 2%...........Relieves joint and muscle soreness
*Hypericum perforatum 6X 0.1%.........Relieves pain
*Millefolium 2X 2%...........................Relieves pain
*Ruta graveolens 4X 2%..................Relieves pain
*Symphytum officinale 4X 0.1%..........Relieves joint pain
*Natural Ingredients
- USES
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS
-
WARNINGS
For external use only. Do not apply over open wounds or broken skin. If symptoms persist or worsen, or if a rash develops, a health professional should be consulted. In rare cases, allergic skin reactions may develop. Do not use if known sensitivity to T-ReliefTM or any of its ingredients exists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. If pregnant or breast-feeding, ask a health professional before use.
-
DIRECTIONS
Twist off cap and remove the foil seal.
Adults and children 4 years and older: Apply generously to affected areas 2 to 3 times daily, or more often if necessary. Massage thoroughly into the skin. If appropriate, mild compression or occlusive bandaging may be applied.
For children under 4,consult your health professional.
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
T-RELIEF
aconitum napellus, arnica montana, baptisia tinctoria root, atropa belladonna, bellis perennis, calendula officinalis flowering top, matricaria recutita, echinacea unspecified, hamamelis virginiana root bark stem bark, hypericum perforatum, achillea millefolium, ruta graveolens flowering top and comfrey root gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62795-7005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 1 [hp_X] in 1 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 4 [hp_X] in 1 g ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 2 [hp_X] in 1 g BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 2 [hp_X] in 1 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 1 g MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 2 [hp_X] in 1 g ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 1 [hp_X] in 1 g HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 2 [hp_X] in 1 g HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 6 [hp_X] in 1 g ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 2 [hp_X] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 2 [hp_X] in 1 g COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 4 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62795-7005-6 1 in 1 CARTON 07/31/2016 1 57 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:62795-7005-5 250 g in 1 BOTTLE; Type 0: Not a Combination Product 07/31/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/31/2016 Labeler - MediNatura Inc (079324099) Establishment Name Address ID/FEI Business Operations Medical Products Laboratories 002290302 manufacture(62795-7005)