| NDC | 44911-0449-1 |
| Set ID | 0d8aa575-3fae-4828-8827-d2561672a91e |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Energique, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
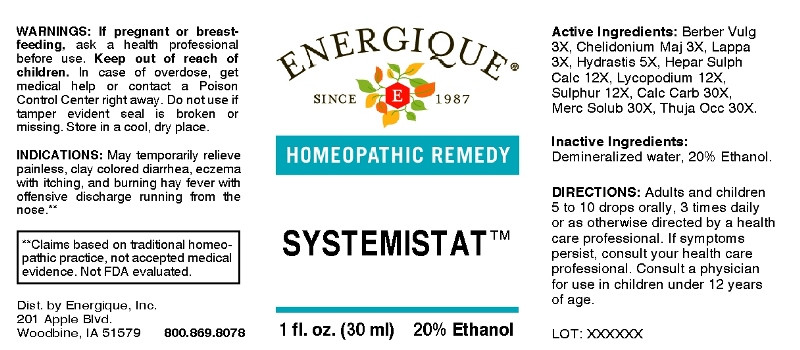
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
SYSTEMISTAT
berberis vulgaris, chelidonium majus, lappa major, hydrastis canadensis, hepar sulphuris calcareum, lycopodium clavatum, sulphur, calcarea carbonica, mercurius solubilis, thuja occidentalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0449 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 3 [hp_X] in 1 mL ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 3 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 5 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_X] in 1 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 30 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0449-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 03/06/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/06/2018 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0449, 44911-0449) , api manufacture(44911-0449) , pack(44911-0449)