| NDC | 29500-9132-1, 29500-9133-1, 29500-9134-1, 29500-9135-1, 29500-9137-1, 29500-9138-1, 29500-9139-1, 29500-9140-1 |
| Set ID | d15c6d1d-fd4f-484b-a30b-9a5d86ff9feb |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Personal Care Products LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART333A |
- Drug FactsActive ingredients
- Purpose
- Keep out of reach of children.
- Use
- Warnings
- When using this product
- Stop use and ask as doctor if
- Directions
- Inactive Ingredients
-
Red Hot Cinnamon - 9132Cheeky Fresh - 9133Love You Berry Much - 9134Sunny Tropicolada - 9135 Fresh breeze - 9137Crazy Coconut - 9138Purrfect Cherry - 9139Citrus Sparkle - 9140 product labels
Red Hot Cinnamon
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
Cheeky Fresh
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
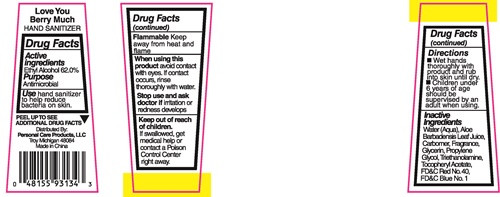
Love You Berry Much
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
Sunny Tropicolada
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
Fresh Breeze
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
Crazy Coconut
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
Purrfect Cherry
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
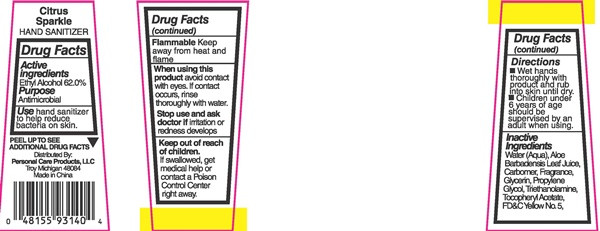
Citrus Sparkle
HAND SANITIZER
PEEL UP TO SEE ADDITIONAL DRUG FACTS
Distrubuted By:
Personak Care Products, LLC
Troy Michigan, 48084
Made in China
-
INGREDIENTS AND APPEARANCE
RED HOT CINNAMON
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9132 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9132-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 CHEEKY FRESH
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9133 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9133-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 LOVE YOU BERRY MUCH
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9134-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 SUNNY TROPICOLADA
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9135-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 FRESH BREEZE
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9137-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 CRAZY COCONUT
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9138-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 PURRFECT CHERRY
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9139-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 CITRUS SPARKLE
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-9140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 26.36 g in 44 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-9140-1 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/23/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/23/2016 Labeler - Personal Care Products LLC (966155082) Registrant - Personal Care Products LLC (966155082)