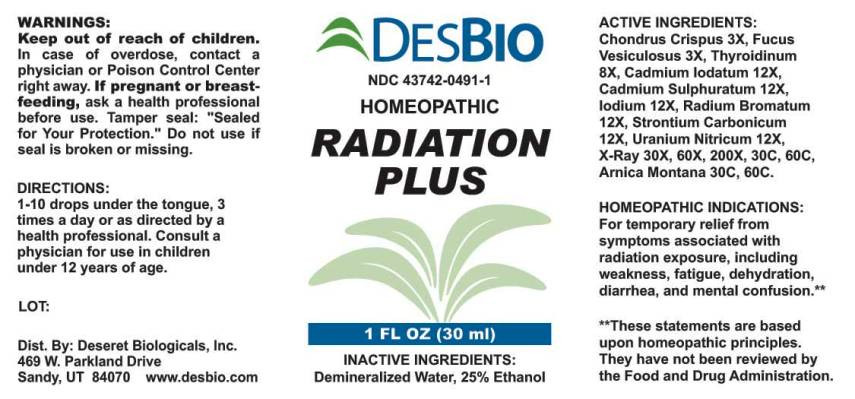
| NDC | 43742-0491-1 |
| Set ID | 2dceb019-eb40-406a-9483-60bc441a5313 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Deseret Biologicals, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENTS:
- HOMEOPATHIC INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN.
- DIRECTIONS:
- HOMEOPATHIC INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
RADIATION PLUS
chondrus crispus, fucus vesiculosus, thyroidinum (suis), cadmium iodatum, cadmium sulphuratum, iodium, uranium nitricum, radium bromatum, strontium carbonicum, x-ray, arnica montana liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-0491 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHONDRUS CRISPUS (UNII: OQS23HUA1X) (CHONDRUS CRISPUS - UNII:OQS23HUA1X) CHONDRUS CRISPUS 3 [hp_X] in 1 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 3 [hp_X] in 1 mL SUS SCROFA THYROID (UNII: 6RV024OAUQ) (SUS SCROFA THYROID - UNII:6RV024OAUQ) SUS SCROFA THYROID 8 [hp_X] in 1 mL CADMIUM IODIDE (UNII: 2F2UPU4KCW) (CADMIUM CATION - UNII:T494FZ4G8G) CADMIUM IODIDE 12 [hp_X] in 1 mL CADMIUM SULFIDE (UNII: 057EZR4Z7Q) (CADMIUM CATION - UNII:T494FZ4G8G) CADMIUM SULFIDE 12 [hp_X] in 1 mL IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 12 [hp_X] in 1 mL URANYL NITRATE HEXAHYDRATE (UNII: 3V057702FY) (URANIUM CATION (6+) - UNII:5PI36AS4G7) URANYL NITRATE HEXAHYDRATE 12 [hp_X] in 1 mL RADIUM BROMIDE (UNII: R74O7T8569) (RADIUM CATION - UNII:05456MVL7T) RADIUM BROMIDE 12 [hp_X] in 1 mL STRONTIUM CARBONATE (UNII: 41YPU4MMCA) (STRONTIUM CATION - UNII:37077S2C93) STRONTIUM CARBONATE 12 [hp_X] in 1 mL ALCOHOL, X-RAY EXPOSED (1000 RAD) (UNII: 6PRJ93602P) (ALCOHOL, X-RAY EXPOSED (1000 RAD) - UNII:6PRJ93602P) ALCOHOL, X-RAY EXPOSED (1000 RAD) 30 [hp_X] in 1 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-0491-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 12/03/2015 08/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/21/2014 08/25/2021 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-0491) , api manufacture(43742-0491) , label(43742-0491) , pack(43742-0491)