| NDC | 67345-0005-0, 67345-0006-0, 67345-0007-0, 67345-0008-0, 67345-0009-0, 67345-0010-0, 67345-0011-0, 67345-0012-0, 67345-0013-0 |
| Set ID | b8a37afb-549b-4d03-aa88-10879ea2af69 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Purminerals |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
- Medicinal Ingredient/Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Non-Medicinal Ingredients/ Inactive Ingredients
Acrylates/C12-22 Alkyl Methacrylate Copolymer, Amodimethicone, Aqua, Bambusa Vulgaris Leaf/Stem Extract, Bis-PEG/PPG-14/14 Dimethicone, Boron Nitride, Butyrospermum Parkii (Shea Butter) Extract, C12-C15 Alkyl Ethylhexanoate, Ceramide AP, Cetyl PEG/PPG-10/1 Dimethicone, Citric Acid, Cyclopentasiloxane, Dextrin, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethiconol, Disteardimonium Hectorite, EDTA, Ethylhexylglycerin, Ferulic Acid, Glucosamine HCL, Hydroxyethylcellulose, Lactic Acid, Laureth-7, Lecithin, Magnesium Sulfate, Mannitol, Pentylene Glycol, Phenoxyethanol, Pisum Sativum (Pea) Extract, Propanediol, Propylene Carbonate, Retinol, Silica Silylate, Sodium Citrate, Sodium Gluconate, Sodium PCA, Sodium Starch Octenylsuccinate, Tocopheryl Acetate, Tribehenin, Triethoxycaprylylsilane, Trihydroxystearin, Trimethylsiloxysilicate, Waltheria Indica Leaf Extract, Xanthan Gum, Zinc Stearate. May Contain: Iron Oxides (CI 77491, CI 77492, CI 77499), Mica, Titanium Dioxide (CI 77891)
- Other
- SPL UNCLASSIFIED SECTION
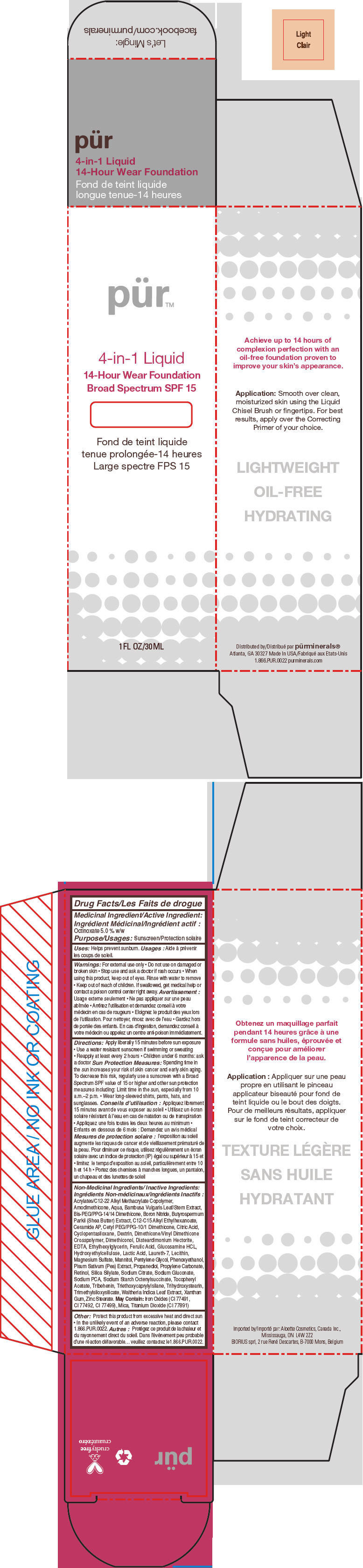
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Light
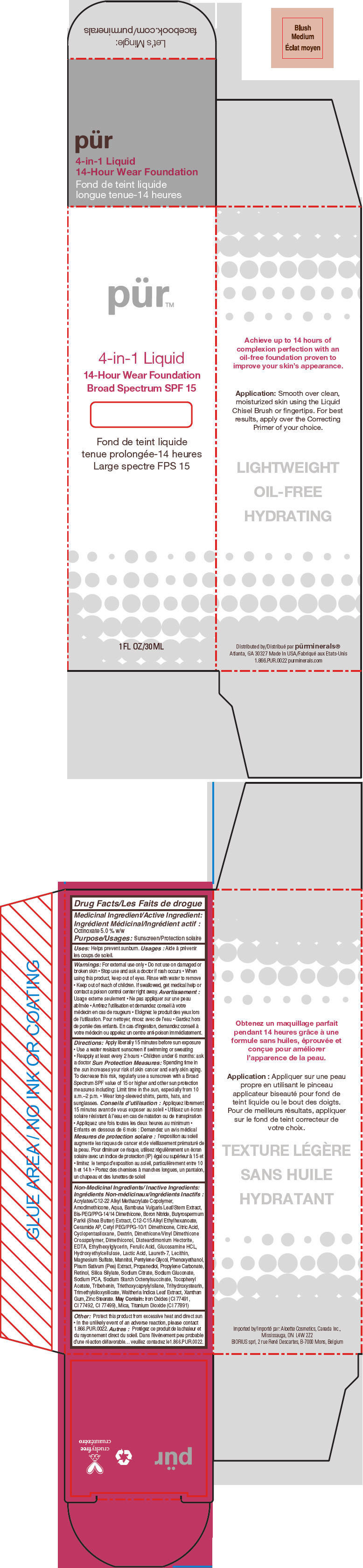
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Blush Medium
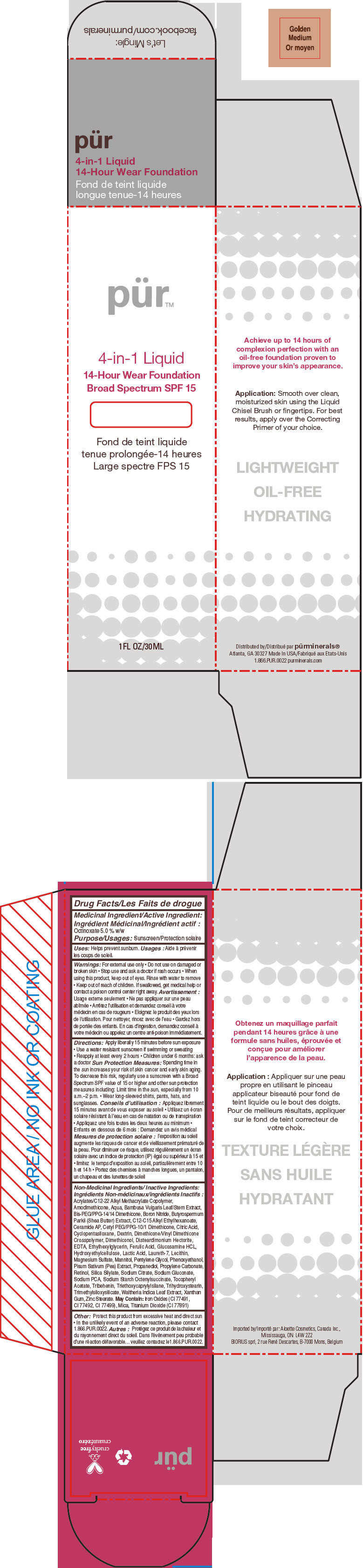
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Golden Medium
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Light Tan
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Tan
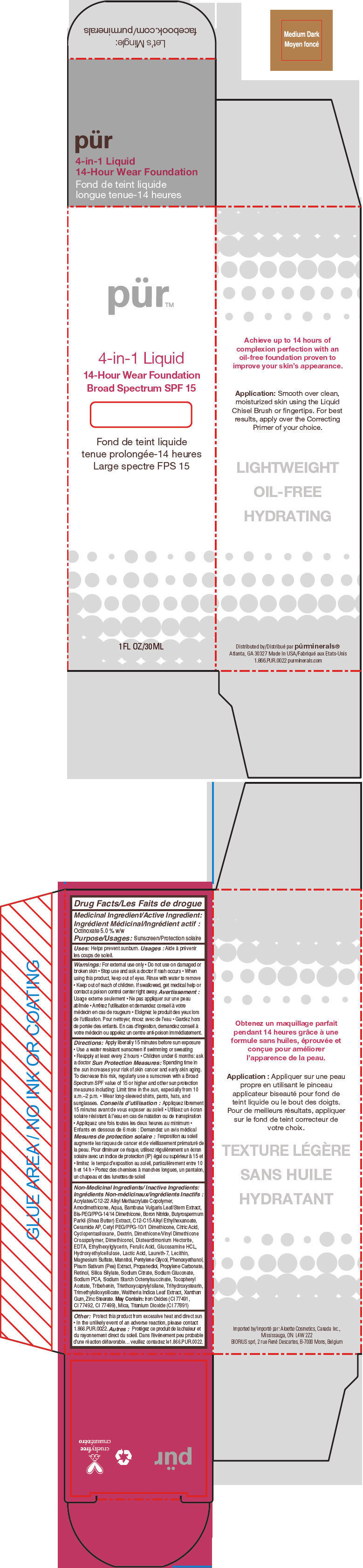
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Medium Dark
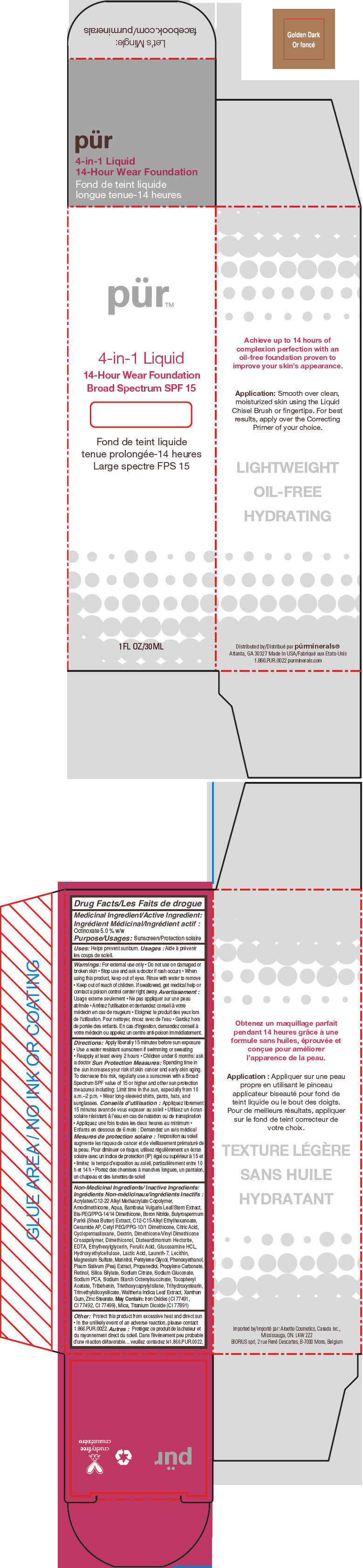
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Golden Dark
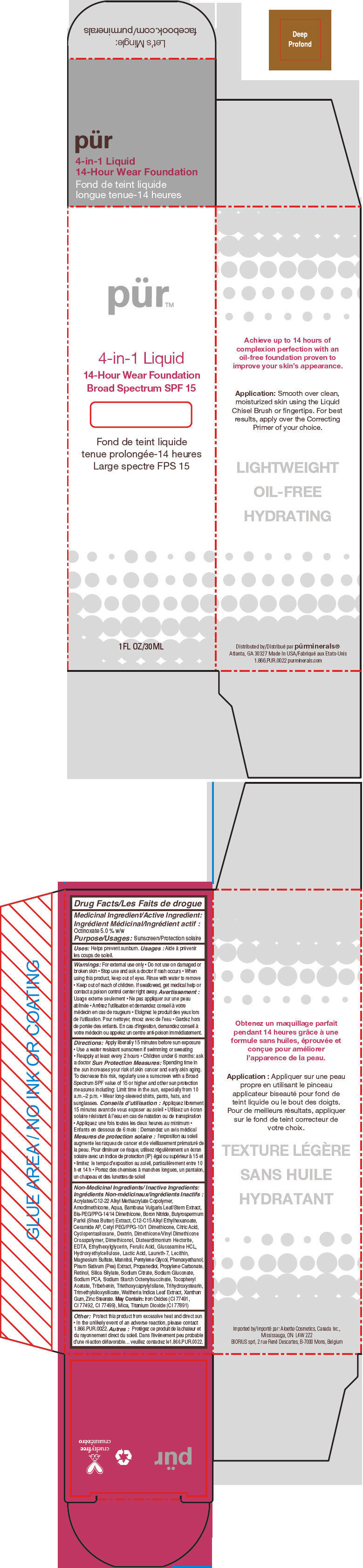
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Deep
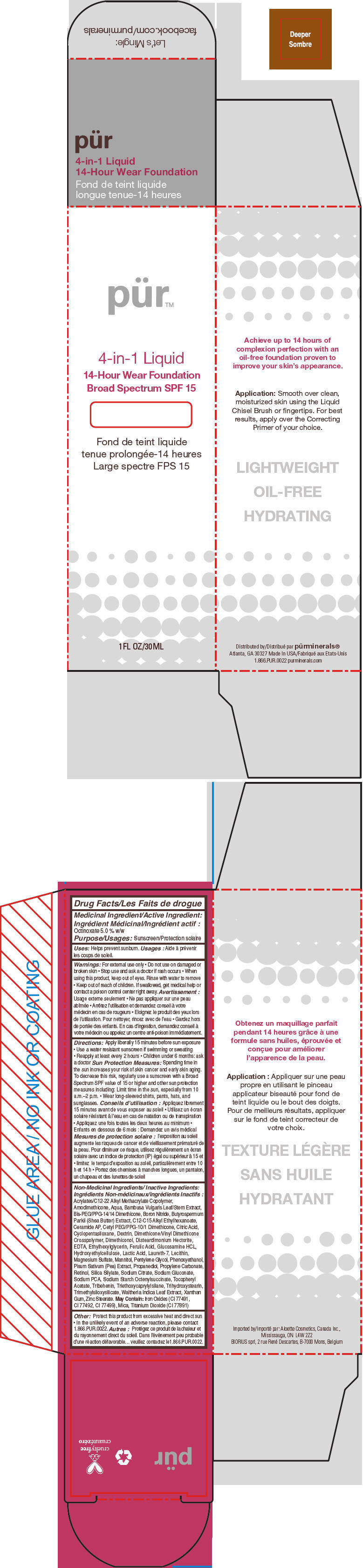
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Deeper
-
INGREDIENTS AND APPEARANCE
PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (LIGHT)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0005-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (BLUSH MEDIUM)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0006-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (GOLDEN MEDIUM)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0007-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (LIGHT TAN)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0008-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (TAN)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0009-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (MEDIUM DARK)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0010-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (GOLDEN DARK)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0011-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (DEEP)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0012-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 PURMINERALS 4-IN-1 14-HOUR WEAR FOUNDATION BROAD SPECTRUM SPF 15 (DEEPER)
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPANEDIOL (UNII: 5965N8W85T) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ZINC STEARATE (UNII: H92E6QA4FV) PHENOXYETHANOL (UNII: HIE492ZZ3T) MANNITOL (UNII: 3OWL53L36A) LAURETH-7 (UNII: Z95S6G8201) TRIBEHENIN (UNII: 8OC9U7TQZ0) SHEANUT OIL (UNII: O88E196QRF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PEA (UNII: W4X7H8GYFM) XANTHAN GUM (UNII: TTV12P4NEE) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM GLUCONATE (UNII: R6Q3791S76) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ICODEXTRIN (UNII: 2NX48Z0A9G) RETINOL (UNII: G2SH0XKK91) WALTHERIA INDICA LEAF (UNII: T69OLD6617) FERULIC ACID (UNII: AVM951ZWST) EDETIC ACID (UNII: 9G34HU7RV0) CERAMIDE AP (UNII: F1X8L2B00J) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0013-0 1 in 1 CARTON 08/01/2012 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2012 Labeler - Purminerals (114450443)