| NDC | 67345-0014-0, 67345-0015-0, 67345-0016-0, 67345-0017-0 |
| Set ID | 3653f951-5f60-4e14-822a-137d2a1fe0d8 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Purminerals |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- Medicinal Ingredient/Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Other
-
Non-Medicinal Ingredients/ Inactive Ingredients
AQUA, ISODODECANE, PHENYL TRIMETHICONE, CYCLOPENTASILOXANE, GLYCERIN, METHYL HYDROGENATED ROSINATE, DIPROPYLENE GLYCOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, CYCLOHEXASILOXANE, ALCOHOL DENAT., CETYL PEG/PPG-10/1 DIMETHICONE, NIACINAMIDE, ORBIGNYA OLEIFERA (BABASSU) SEED OIL, BUTYLENE GLYCOL, PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, MAGNESIUM SULFATE, TRIETHOXYCAPRYLYLSILANE, STEARALKONIUM HECTORITE, SORBITAN ISOSTEARATE, POLYGLYCERYL-4 ISOSTEARATE, ALUMINUM HYDROXIDE, HEXYL LAURATE, CAPRYLYL GLYCOL, PROPYLENE CARBONATE, STEARIC ACID, PHENOXYETHANOL, FRAGRANCE (PARFUM), ADANSONIA DIGITATA SEED OIL, TOCOPHERYL ACETATE, SODIUM HYALURONATE, SODIUM STARCH OCTENYLSUCCINATE, 1,2-HEXANEDIOL, ADENOSINE, DISODIUM EDTA, MANGIFERA INDICA (MANGO) SEED OIL, LACTIC ACID, BUTYROSPERMUM PARKII (SHEA) BUTTER, HIPPOPHAE RHAMNOIDES OIL, RETINOL, CERAMIDE AP. MAY CONTAIN: TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, CI 77492, CI 77499)
- SPL UNCLASSIFIED SECTION
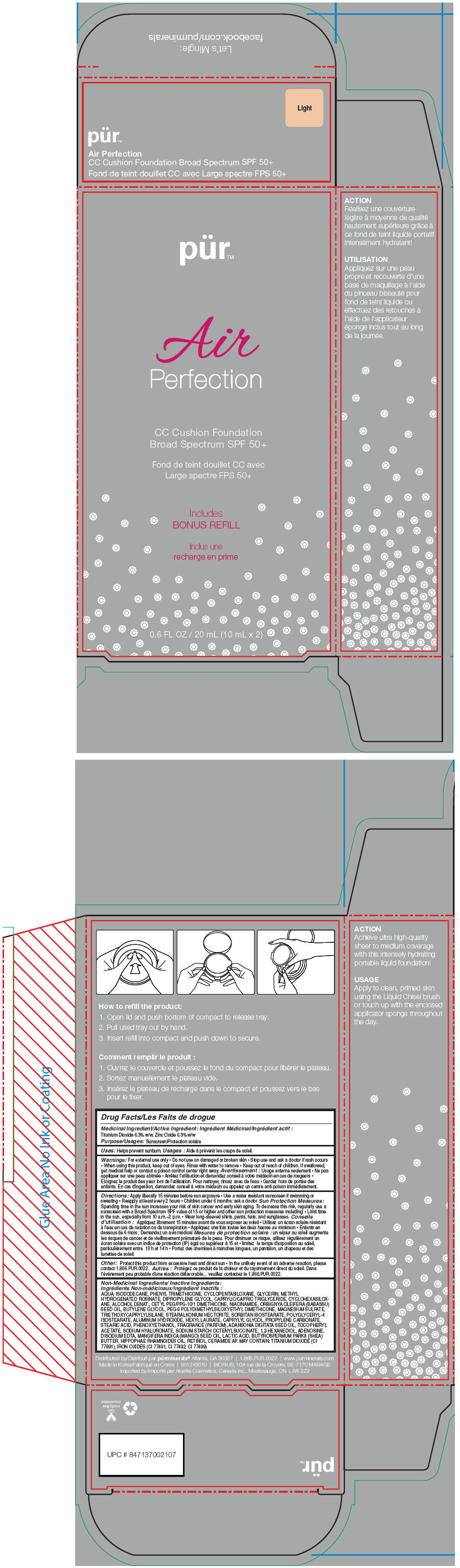
- PRINCIPAL DISPLAY PANEL - 20 mL Cartridge Carton - Light
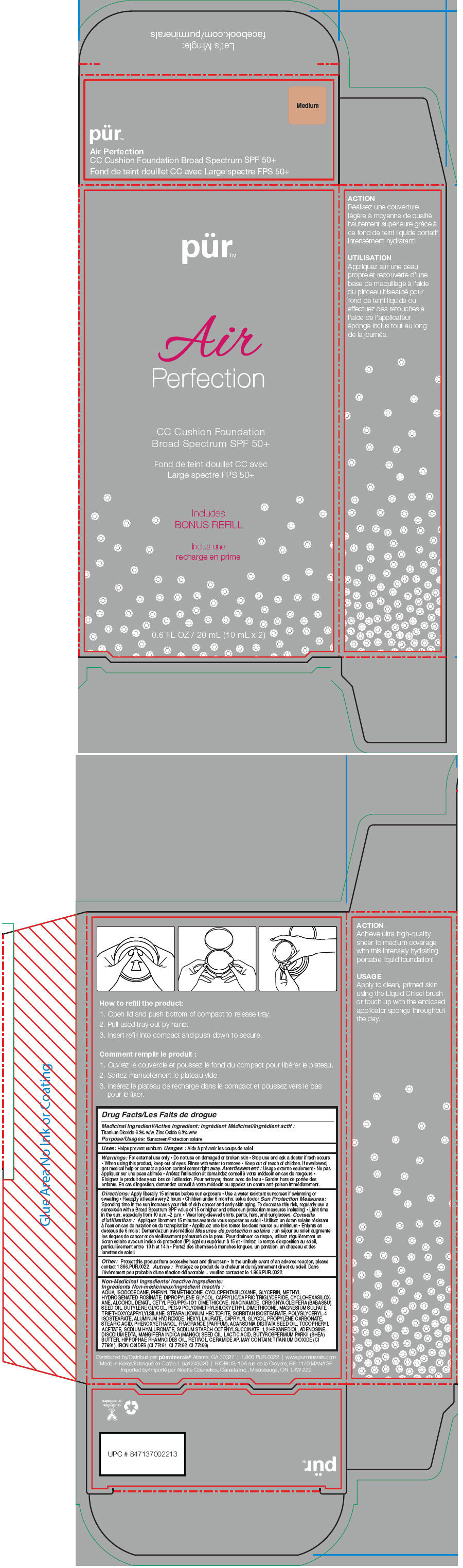
- PRINCIPAL DISPLAY PANEL - 20 mL Cartridge Carton - Medium
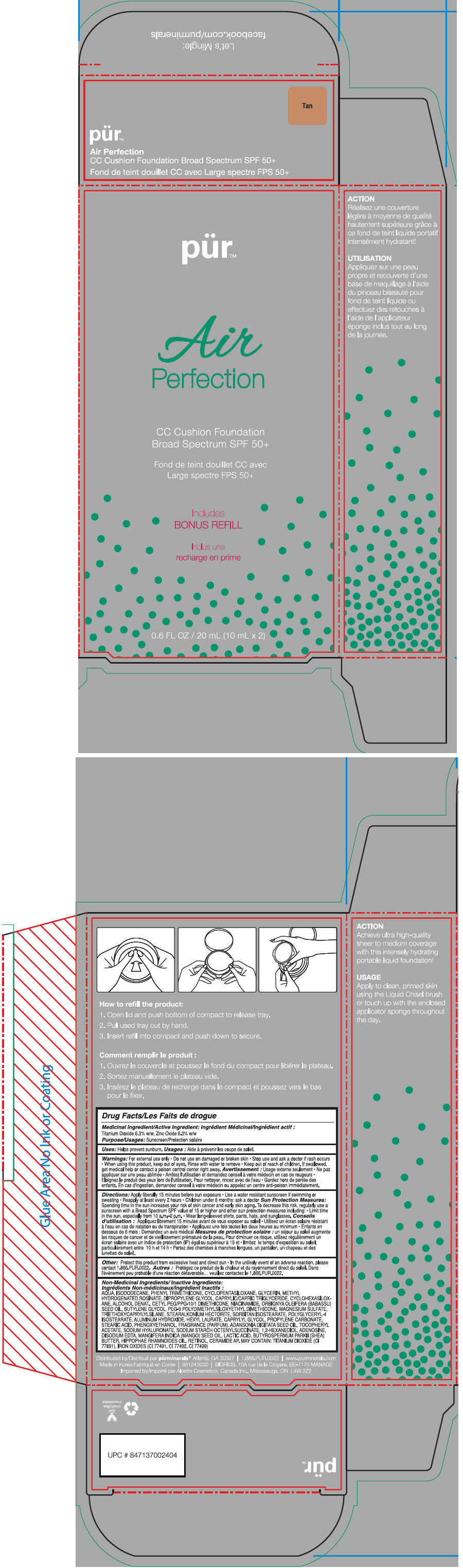
- PRINCIPAL DISPLAY PANEL - 20 mL Cartridge Carton - Tan
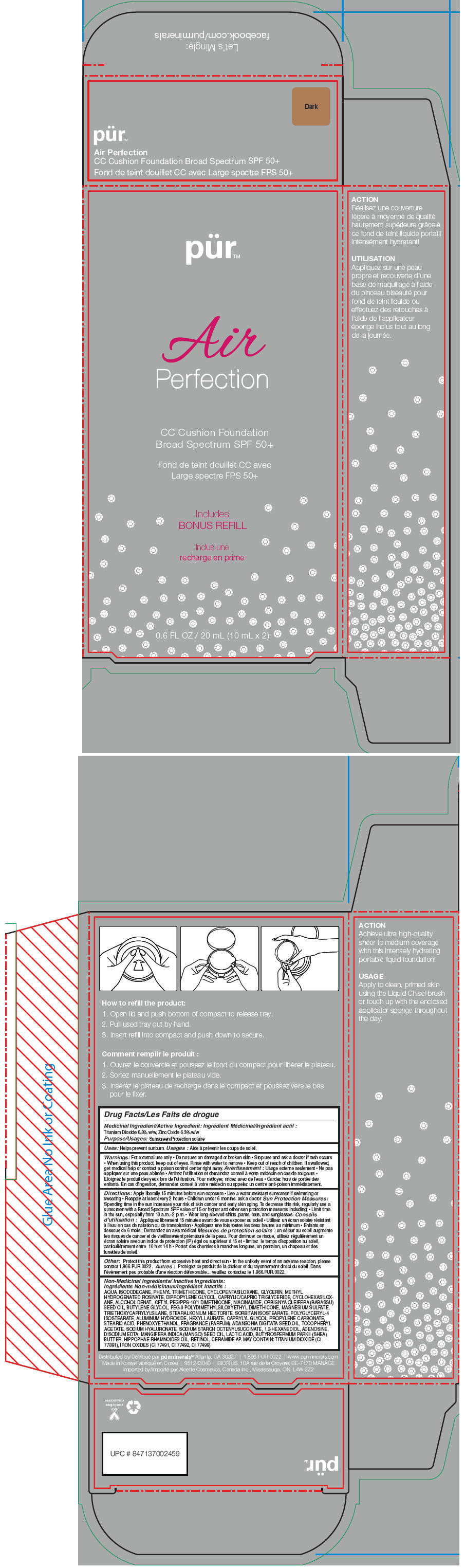
- PRINCIPAL DISPLAY PANEL - 20 mL Cartridge Carton - Dark
-
INGREDIENTS AND APPEARANCE
PUR AIR PERFECTION CC CUSHION FOUNDATION BROAD SPECTRUM SPF 50 PLUS (LIGHT)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 63 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isododecane (UNII: A8289P68Y2) Phenyl Trimethicone (UNII: DR0K5NOJ4R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glycerin (UNII: PDC6A3C0OX) Methyl Hydrogenated Rosinate (UNII: 13DHA19W9N) Dipropylene Glycol (UNII: E107L85C40) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Cyclomethicone 6 (UNII: XHK3U310BA) Alcohol (UNII: 3K9958V90M) Cetyl PEG/PPG-10/1 Dimethicone (HLB 4) (UNII: 8INO2K35FA) Niacinamide (UNII: 25X51I8RD4) Babassu Oil (UNII: 8QSB4M5477) Butylene Glycol (UNII: 3XUS85K0RA) PEG-9 Polydimethylsiloxyethyl Dimethicone (UNII: TYP81E471F) Magnesium Sulfate (UNII: DE08037SAB) Triethoxycaprylylsilane (UNII: LDC331P08E) Stearalkonium Hectorite (UNII: OLX698AH5P) Sorbitan Isostearate (UNII: 01S2G2C1E4) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Hexyl Laurate (UNII: 4CG9F9W01Q) Caprylyl Glycol (UNII: 00YIU5438U) Propylene Carbonate (UNII: 8D08K3S51E) Stearic Acid (UNII: 4ELV7Z65AP) Phenoxyethanol (UNII: HIE492ZZ3T) Adansonia Digitata Seed Oil (UNII: 77MKL7AR5I) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Hyaluronate Sodium (UNII: YSE9PPT4TH) 1,2-Hexanediol (UNII: TR046Y3K1G) Adenosine (UNII: K72T3FS567) Edetate Disodium (UNII: 7FLD91C86K) Mango Seed Oil (UNII: K8LOR30915) Lactic Acid (UNII: 33X04XA5AT) Shea Butter (UNII: K49155WL9Y) Hippophae Rhamnoides Seed Oil (UNII: T53SBG6741) Retinol (UNII: G2SH0XKK91) Ceramide 6 Ii (UNII: F1X8L2B00J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0014-0 1 in 1 CARTON 1 10 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2015 PUR AIR PERFECTION CC CUSHION FOUNDATION BROAD SPECTRUM SPF 50 PLUS (MEDIUM)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 63 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isododecane (UNII: A8289P68Y2) Phenyl Trimethicone (UNII: DR0K5NOJ4R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glycerin (UNII: PDC6A3C0OX) Methyl Hydrogenated Rosinate (UNII: 13DHA19W9N) Dipropylene Glycol (UNII: E107L85C40) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Cyclomethicone 6 (UNII: XHK3U310BA) Alcohol (UNII: 3K9958V90M) Cetyl PEG/PPG-10/1 Dimethicone (HLB 4) (UNII: 8INO2K35FA) Niacinamide (UNII: 25X51I8RD4) Babassu Oil (UNII: 8QSB4M5477) Butylene Glycol (UNII: 3XUS85K0RA) PEG-9 Polydimethylsiloxyethyl Dimethicone (UNII: TYP81E471F) Magnesium Sulfate (UNII: DE08037SAB) Triethoxycaprylylsilane (UNII: LDC331P08E) Stearalkonium Hectorite (UNII: OLX698AH5P) Sorbitan Isostearate (UNII: 01S2G2C1E4) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Hexyl Laurate (UNII: 4CG9F9W01Q) Caprylyl Glycol (UNII: 00YIU5438U) Propylene Carbonate (UNII: 8D08K3S51E) Stearic Acid (UNII: 4ELV7Z65AP) Phenoxyethanol (UNII: HIE492ZZ3T) Adansonia Digitata Seed Oil (UNII: 77MKL7AR5I) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Hyaluronate Sodium (UNII: YSE9PPT4TH) 1,2-Hexanediol (UNII: TR046Y3K1G) Adenosine (UNII: K72T3FS567) Edetate Disodium (UNII: 7FLD91C86K) Mango Seed Oil (UNII: K8LOR30915) Lactic Acid (UNII: 33X04XA5AT) Shea Butter (UNII: K49155WL9Y) Hippophae Rhamnoides Seed Oil (UNII: T53SBG6741) Retinol (UNII: G2SH0XKK91) Ceramide 6 Ii (UNII: F1X8L2B00J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0015-0 1 in 1 CARTON 1 10 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2015 PUR AIR PERFECTION CC CUSHION FOUNDATION BROAD SPECTRUM SPF 50 PLUS (TAN)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 63 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isododecane (UNII: A8289P68Y2) Phenyl Trimethicone (UNII: DR0K5NOJ4R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glycerin (UNII: PDC6A3C0OX) Methyl Hydrogenated Rosinate (UNII: 13DHA19W9N) Dipropylene Glycol (UNII: E107L85C40) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Cyclomethicone 6 (UNII: XHK3U310BA) Alcohol (UNII: 3K9958V90M) Cetyl PEG/PPG-10/1 Dimethicone (HLB 4) (UNII: 8INO2K35FA) Niacinamide (UNII: 25X51I8RD4) Babassu Oil (UNII: 8QSB4M5477) Butylene Glycol (UNII: 3XUS85K0RA) PEG-9 Polydimethylsiloxyethyl Dimethicone (UNII: TYP81E471F) Magnesium Sulfate (UNII: DE08037SAB) Triethoxycaprylylsilane (UNII: LDC331P08E) Stearalkonium Hectorite (UNII: OLX698AH5P) Sorbitan Isostearate (UNII: 01S2G2C1E4) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Hexyl Laurate (UNII: 4CG9F9W01Q) Caprylyl Glycol (UNII: 00YIU5438U) Propylene Carbonate (UNII: 8D08K3S51E) Stearic Acid (UNII: 4ELV7Z65AP) Phenoxyethanol (UNII: HIE492ZZ3T) Adansonia Digitata Seed Oil (UNII: 77MKL7AR5I) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Hyaluronate Sodium (UNII: YSE9PPT4TH) 1,2-Hexanediol (UNII: TR046Y3K1G) Adenosine (UNII: K72T3FS567) Edetate Disodium (UNII: 7FLD91C86K) Mango Seed Oil (UNII: K8LOR30915) Lactic Acid (UNII: 33X04XA5AT) Shea Butter (UNII: K49155WL9Y) Hippophae Rhamnoides Seed Oil (UNII: T53SBG6741) Retinol (UNII: G2SH0XKK91) Ceramide 6 Ii (UNII: F1X8L2B00J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0016-0 1 in 1 CARTON 1 10 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2015 PUR AIR PERFECTION CC CUSHION FOUNDATION BROAD SPECTRUM SPF 50 PLUS (DARK)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 63 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isododecane (UNII: A8289P68Y2) Phenyl Trimethicone (UNII: DR0K5NOJ4R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glycerin (UNII: PDC6A3C0OX) Methyl Hydrogenated Rosinate (UNII: 13DHA19W9N) Dipropylene Glycol (UNII: E107L85C40) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Cyclomethicone 6 (UNII: XHK3U310BA) Alcohol (UNII: 3K9958V90M) Cetyl PEG/PPG-10/1 Dimethicone (HLB 4) (UNII: 8INO2K35FA) Niacinamide (UNII: 25X51I8RD4) Babassu Oil (UNII: 8QSB4M5477) Butylene Glycol (UNII: 3XUS85K0RA) PEG-9 Polydimethylsiloxyethyl Dimethicone (UNII: TYP81E471F) Magnesium Sulfate (UNII: DE08037SAB) Triethoxycaprylylsilane (UNII: LDC331P08E) Stearalkonium Hectorite (UNII: OLX698AH5P) Sorbitan Isostearate (UNII: 01S2G2C1E4) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Hexyl Laurate (UNII: 4CG9F9W01Q) Caprylyl Glycol (UNII: 00YIU5438U) Propylene Carbonate (UNII: 8D08K3S51E) Stearic Acid (UNII: 4ELV7Z65AP) Phenoxyethanol (UNII: HIE492ZZ3T) Adansonia Digitata Seed Oil (UNII: 77MKL7AR5I) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Hyaluronate Sodium (UNII: YSE9PPT4TH) 1,2-Hexanediol (UNII: TR046Y3K1G) Adenosine (UNII: K72T3FS567) Edetate Disodium (UNII: 7FLD91C86K) Mango Seed Oil (UNII: K8LOR30915) Lactic Acid (UNII: 33X04XA5AT) Shea Butter (UNII: K49155WL9Y) Hippophae Rhamnoides Seed Oil (UNII: T53SBG6741) Retinol (UNII: G2SH0XKK91) Ceramide 6 Ii (UNII: F1X8L2B00J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0017-0 1 in 1 CARTON 1 10 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2015 Labeler - Purminerals (114450443)