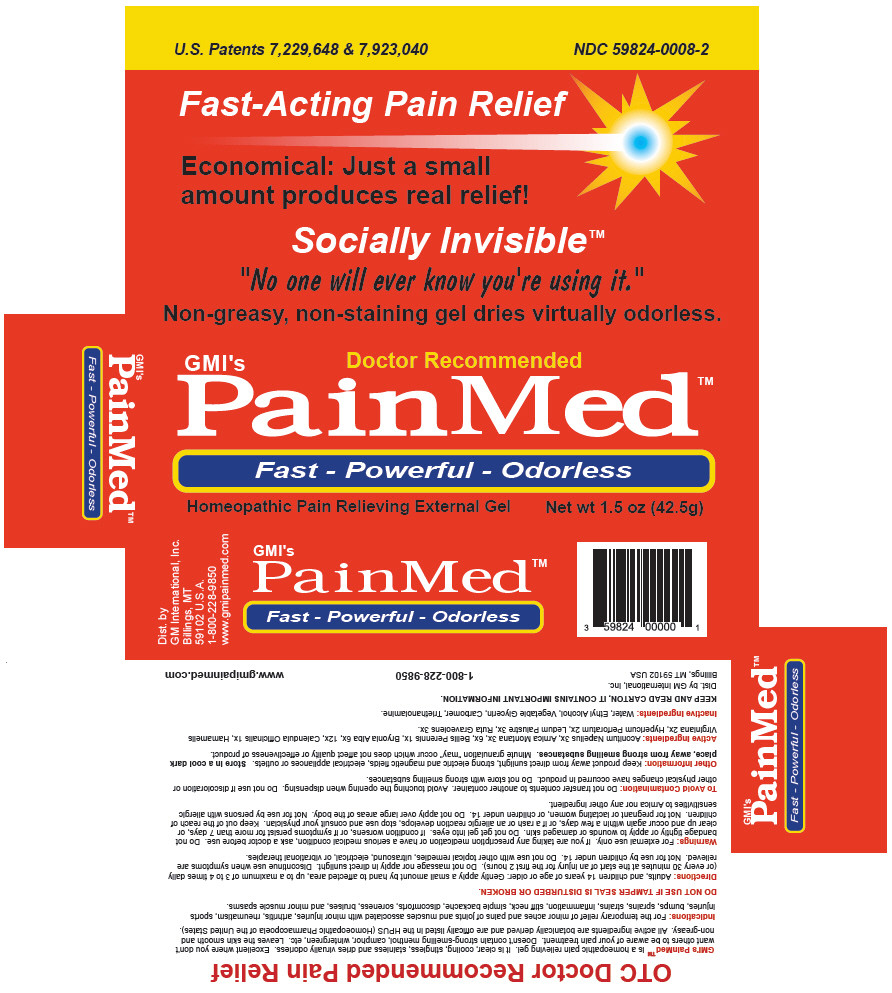
| NDC | 59824-0008-2 |
| Set ID | cf85b8db-ba3c-4478-9456-90d7adb65286 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | G M International, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Indications
- SPL UNCLASSIFIED SECTION
-
Directions
Adults, and children 14 years of age or older: Gently apply a small amount by hand to affected area, up to a maximum of 3 to 4 times daily (or every 30 minutes at the start of an injury for the first 2 hours). Do not massage nor apply in direct sunlight. Discontinue use when symptoms are relieved. Not for use by children under 14. Do not use with other topical remedies, ultrasound, electrical, or vibrational therapies.
-
Warnings
For external use only.
If you are taking any prescription medication or have a serious medical condition, ask a doctor before use.
- Active Ingredients
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL - 42.5g Tube Carton
-
INGREDIENTS AND APPEARANCE
PAINMED HOMEOPATHIC PAIN RELIEVING EXTERNAL
aconitum napellus, arnica montana, bellis perennis, bryonia alba root, calendula officinalis flowering top, hamamelis virginiana root bark/stem bark, hypericum perforatum, ledum palustre twig, and ruta graveolens flowering top gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59824-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 3 [hp_X] in 1 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 6 [hp_X] in 1 g BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 1 [hp_X] in 1 g BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 12 [hp_X] in 1 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 1 g HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 2 [hp_X] in 1 g HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 2 [hp_X] in 1 g RHODODENDRON TOMENTOSUM LEAFY TWIG (UNII: 877L01IZ0P) (RHODODENDRON TOMENTOSUM LEAFY TWIG - UNII:877L01IZ0P) RHODODENDRON TOMENTOSUM LEAFY TWIG 3 [hp_X] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 3 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59824-0008-2 1 in 1 BOX 08/01/2003 1 42.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 08/01/2003 Labeler - G M International, Inc. (616177916) Establishment Name Address ID/FEI Business Operations Cosmetic Creations Inc. 079375497 MANUFACTURE(59824-0008) , LABEL(59824-0008)