| NDC | 49726-012-02 |
| Set ID | 863fb393-0af0-4ee1-8c78-b7ba3c4f2ba4 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Hello Life, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
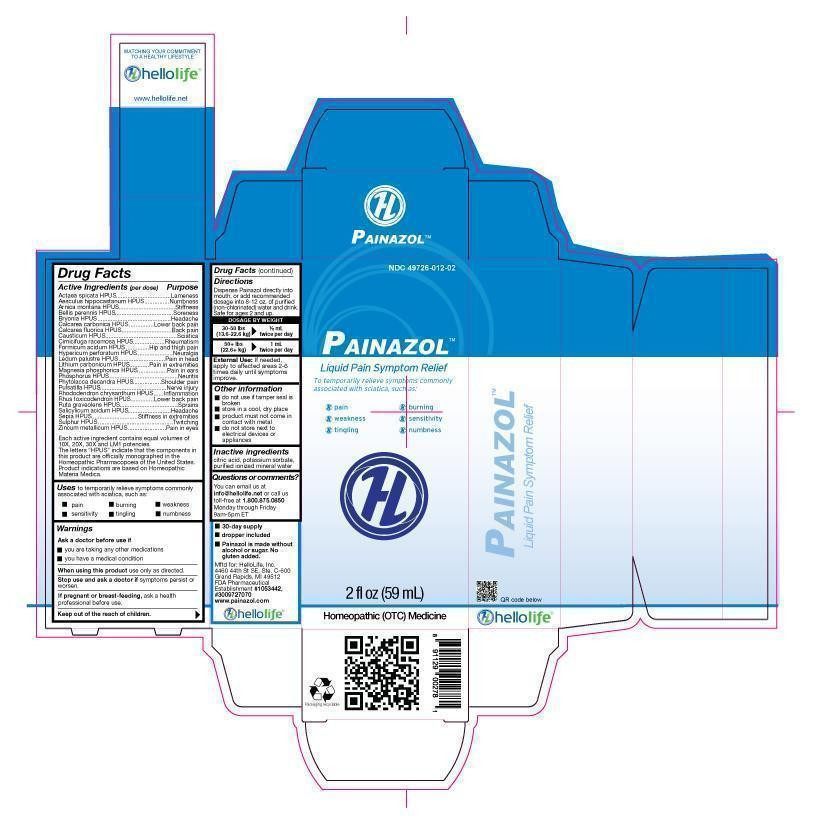
Active Ingredient (per dose)
Actaea spicata HPUS, Aesculus hippocastanum HPUS, Arnica Montana HPUS, Bellis perennis HPUS, Bryonia HPUS, Calcarea carbonica HPUS, Calcarea fluorica HPUS, Causticum HPUS, Cimicifuga racemosa HPUS, Formicum acidum HPUS, Hypericum perforatum HPUS, Ledum palustre HPUS, Lithium carbonicum HPUS, Magnesia phosphorica HPUS, Phosphorus HPUS, Phytolacca decandra HPUS, Pulsatilla HPUS, Rhododendron chrysanthum HPUS, Rhus toxicodendron HPUS, Ruta graveolens HPUS, Salicylicum acidum HPUS, Sepia HPUS, Sulphur HPUS, Zincum metallicum HPUS
Each active ingredient contains equal volumes of 10X, 20X, 30X and LM1 potencies. The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. Product indications are based on Homeopathic Materia Medica. -
Purpose
Actaea spicata HPUS.......................................................Lameness
Aesculus hippocastanum HPUS........................................Numbness
Arnica Montana HPUS......................................................Stiffness
Bellis perennis HPUS.......................................................Soreness
Bryonia HPUS.................................................................Headache
Calcarea carbonica HPUS................................................Lower back pain
Calcarea fluorica HPUS....................................................Back pain
Causticum HPUS............................................................Sciatica
Cimicifuga racemosa HPUS.............................................Rheumatism
Formicum acidum HPUS..................................................Hip and thigh pain
Hypericum perforatum HPUS............................................Neuralgia
Ledum palustre HPUS......................................................Pain in head
Lithium carbonicum HPUS................................................Pain in extremities
Magnesia phosphorica HPUS...........................................Pain in ears
Phosphorus HPUS...........................................................Neuritis
Phytolacca decandra HPUS..............................................Shoulder pain
Pulsatilla HPUS...............................................................Nerve injury
Rhododendron chrysanthum HPUS....................................Inflammation
Rhus toxicodendron HPUS................................................Lower back pain
Ruta graveolens HPUS.....................................................Sprains
Salicylicum acidum HPUS................................................Headaches
Sepia HPUS....................................................................Stiffness in extremities
Sulphur HPUS..................................................................Twitching
Zincum metallicum HPUS..................................................Pain in eyes
- Warnings
-
Directions
Dispense Painazol directly into mouth, or add recommended dosage to 8-12 oz purified (non-chlorinated water) and drink.
Dosage by Weight:
30-50 lbs (13.6-22.6 kg) 1/2 mL twice per day
50+ lbs (22.6+ kg) 1 mL twice per day.
External Use: If needed, apply to affected areas 2-6 times daily until symptoms improve.
- Other Information
- Inactive ingredients
- Questions or comments
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAINAZOL PAIN RELIEF
actaea spicata, aesculus hippocastanum, arnica montana, bellis perennis, bryonia alba, calcarea carbonica, calcarea fluorica, causticum, cimicifuga racemosa, formicum acidum, hypericum perforatum, ledum paulstre, lithium carbonicum, magnesia phosphorica, phosphorus, phytolacca decandra, pulsatilla, rhododendron chrysanthum, rhus toxicodendron, ruta graveolens, salicylicum acidum, sepia, sulphur, zincum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49726-012 Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTAEA SPICATA ROOT (UNII: 3FU86L9OS0) (ACTAEA SPICATA ROOT - UNII:3FU86L9OS0) ACTAEA SPICATA ROOT 10 [hp_X] in 59 mL AESCULUS HIPPOCASTANUM FLOWER (UNII: KK0Z92II8M) (AESCULUS HIPPOCASTANUM FLOWER - UNII:KK0Z92II8M) AESCULUS HIPPOCASTANUM FLOWER 10 [hp_X] in 59 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 10 [hp_X] in 59 mL BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 10 [hp_X] in 59 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 59 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 10 [hp_X] in 59 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 10 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 59 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 10 [hp_X] in 59 mL FORMIC ACID (UNII: 0YIW783RG1) (FORMIC ACID - UNII:0YIW783RG1) FORMIC ACID 10 [hp_X] in 59 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 10 [hp_X] in 59 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 10 [hp_X] in 59 mL LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 10 [hp_X] in 59 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 10 [hp_X] in 59 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 10 [hp_X] in 59 mL RHODODENDRON AUREUM LEAF (UNII: IV92NQJ73U) (RHODODENDRON AUREUM LEAF - UNII:IV92NQJ73U) RHODODENDRON AUREUM LEAF 10 [hp_X] in 59 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 59 mL RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 10 [hp_X] in 59 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 [hp_X] in 59 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 10 [hp_X] in 59 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 59 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49726-012-02 1 in 1 CARTON 05/20/2010 1 59 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/20/2010 Labeler - Hello Life, Inc. (065619378) Establishment Name Address ID/FEI Business Operations Hello Life, Inc. 065619378 relabel(49726-012) , repack(49726-012) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(49726-012)