| NDC | 43689-0023-1, 43689-0023-2 |
| Set ID | 4e971e2d-9688-4347-bdbc-14c23c4e4d4f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | The Magni Company |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
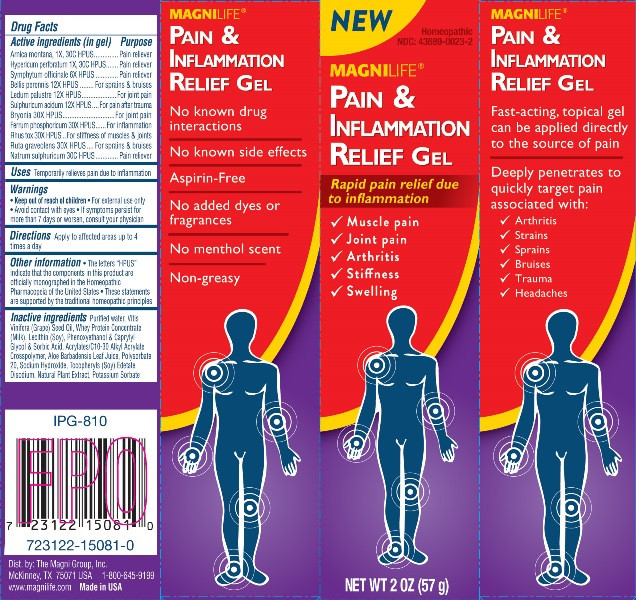
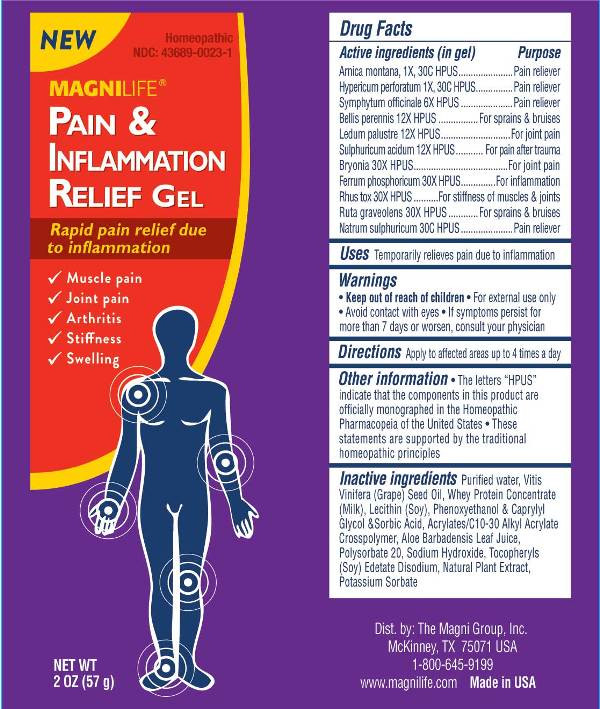
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
• Keep out of reach of children. • For external use only.
• Avoid contact with eyes. • If symptoms persist for
more than 7 days or worsen, consult your physician.
OTHER INFORMATION:
The letters "HPUS" indicate that the components in this product are officially monographed
in the Homeopathic Pharmacopeia of the United States.
These statements are supported by the traditional homeopathic principles.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
-
INACTIVE INGREDIENTS:
Purified Water, Vitis Vinifera (Grape) Seed Oil, Whey Protein Concentrate (Milk), Lecithin (Soy), Phenoxyethanol & Caprylyl Glycol & Sorbic Acid, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Polysorbate 20, Sodium Hydroxide, Tocopheryls (Soy), Edetate Disodium, Natural Plant Extract, Potassium Sorbate
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
PAIN AND INFLAMMATION
arnica montana, hypericum perforatum, symphytum officinale, bellis perennis, ledum palustre, sulphuricum acidum, bryonia, ferrum phosphoricum, rhus tox, ruta graveolens, natrum sulphuricum gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43689-0023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 1 [hp_X] in 1 g COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 1 g BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 12 [hp_X] in 1 g LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 12 [hp_X] in 1 g SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 12 [hp_X] in 1 g BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_X] in 1 g FERRUM PHOSPHORICUM (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERRUM PHOSPHORICUM 30 [hp_X] in 1 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 30 [hp_X] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 30 [hp_X] in 1 g SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 30 [hp_C] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GRAPE SEED OIL (UNII: 930MLC8XGG) WHEY (UNII: 8617Z5FMF6) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBIC ACID (UNII: X045WJ989B) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM HYDROXIDE (UNII: 55X04QC32I) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) EDETATE DISODIUM (UNII: 7FLD91C86K) OREGANO (UNII: 0E5AT8T16U) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43689-0023-2 1 in 1 CARTON 06/02/2016 1 NDC:43689-0023-1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/02/2016 Labeler - The Magni Company (113501902) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43689-0023) , api manufacture(43689-0023) , label(43689-0023) , pack(43689-0023)