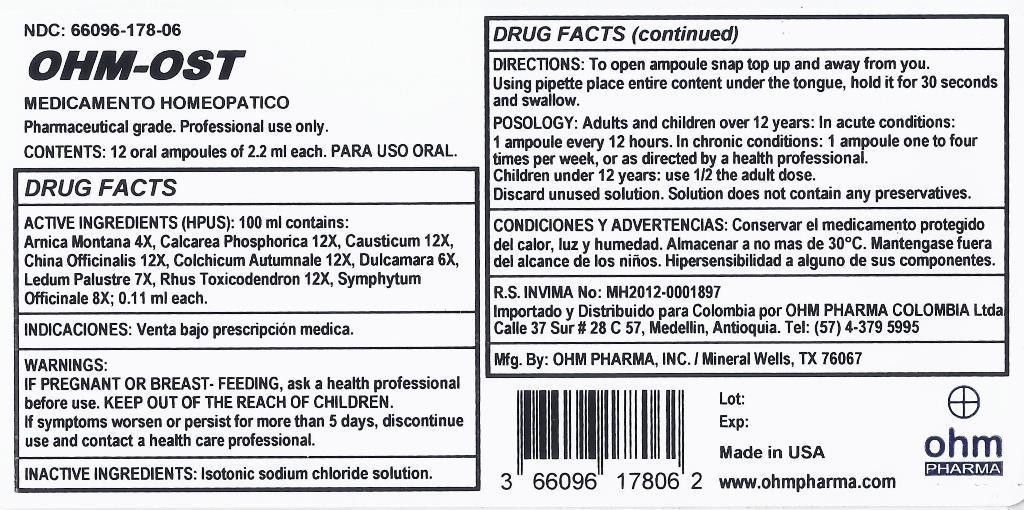
| NDC | 66096-178-06 |
| Set ID | 66ff847d-3d89-4a4b-97fa-1fee11cca2ad |
| Category | HUMAN OTC DRUG LABEL |
| Packager | OHM PHARMA INC. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
DIRECTIONS: To open ampoule snap top up and away from you. Using pipette place entire content under the tongue, hold it for 30 seconds and swallow.
POSOLOGY: Adults and children over 12 years: In acute conditions: 1 ampoule every 12 hours. In chronic conditions: 1 ampoule one to four times per week, or as directed by a health professional.
Children under 12 years use 1/2 the adult dose.
Discard unused solution. Solution does not contain any preservatives.
Not for injection.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OHM OSTEOARTICULAR TERRAIN
arnica montana, calcarea phosphorica, causticum, china officinalis, colchicum autumnale, dulcamara, ledum palustre, rhus toxicodendron, symphytum officinale liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66096-178 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 4 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] in 1 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 12 [hp_X] in 1 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 12 [hp_X] in 1 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 6 [hp_X] in 1 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 7 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 1 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 8 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66096-178-06 12 in 1 BOX 04/23/2015 1 2.2 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/23/2015 Labeler - OHM PHARMA INC. (030572478) Registrant - OHM PHARMA INC. (030572478) Establishment Name Address ID/FEI Business Operations OHM PHARMA INC. 030572478 manufacture(66096-178)