| NDC | 50242-043-14, 50242-073-01, 50242-074-01, 50242-075-01, 50242-076-01 |
| Set ID | 139d2038-e6a9-4ab1-ab00-aa7d8aa8df5f |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Genentech, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | NDA020522 |
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Nutropin AQ safely and effectively. See full prescribing information for Nutropin AQ.
Nutropin AQ (somatropin) injection, for subcutaneous use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
Nutropin AQ is a recombinant human growth hormone indicated for:
- Pediatric Patients: Treatment of children with growth failure due to growth hormone deficiency (GHD), idiopathic short stature (ISS), Turner syndrome (TS), and chronic kidney disease (CKD) up to the time of renal transplantation (1.1).
- Adult Patients: Treatment of adults with either childhood-onset or adult-onset GHD (1.2).
DOSAGE AND ADMINISTRATION
Nutropin AQ should be administered subcutaneously (2).
Injection sites should always be rotated to avoid lipoatrophy (2.3).
- Pediatric GHD:
- Up to 0.3 mg/kg/week (2.1)
- Pubertal Patients:
- Up to 0.7 mg/kg/week (2.1)
- Idiopathic Short Stature:
- Up to 0.3 mg/kg/week (2.1)
- Chronic Kidney Disease:
- Up to 0.35 mg/kg/week (2.1)
- Turner Syndrome:
- Up to 0.375 mg/kg/week (2.1)
-
Adult GHD: Either a non-weight based or weight-based dosing regimen may be followed, with doses adjusted based on treatment response and IGF-I concentrations (2.2).
Non-weight-based: A starting dose of approximately 0.2 mg/day (range 0.15–0.3 mg/day) increased gradually every 1–2 months by increments of approximately 0.1–0.2 mg/day.
Weight-based: Initiate from not more than 0.006 mg/kg/day; the dose may be increased up to a maximum of 0.025 mg/kg/day in patients ≤ 35 years old or 0.0125 mg/kg/day in patients > 35 years old.
DOSAGE FORMS AND STRENGTHS
Nutropin AQ is a sterile liquid available in the following pen cartridge and NuSpin forms (3):
- Pen Cartridge: 10 mg/2 mL (yellow color band), and 20 mg/2 mL (purple color band).
- NuSpin: 5 mg/2 mL (clear device), 10 mg/2 mL (green device), and 20 mg/2 mL (blue device).
CONTRAINDICATIONS
- Acute critical illness (4).
- Children with Prader-Willi syndrome (PWS) who are severely obese or have severe respiratory impairment – reports of sudden death (4).
- Active malignancy (4).
- Hypersensitivity to somatropin or excipients (4).
- Active proliferative or severe non-proliferative diabetic retinopathy (4).
- Children with closed epiphysis (4).
WARNINGS AND PRECAUTIONS
- Acute critical illness: Evaluate potential benefit of treatment continuation against potential risk (5.1).
- PWS: Evaluate for signs of upper airway obstruction and sleep apnea before initiating therapy. Discontinue treatment if these signs occur. (5.2).
- Neoplasm: Monitor patients with preexisting tumors for progression or reoccurrence. Increased risk of a second neoplasm in childhood cancer survivors treated with somatropin - in particular meningiomas in patients treated with radiation to the head for their first neoplasm. (5.3).
- Impaired glucose tolerance (IGT) and Diabetes Mellitus (DM): Periodically monitor glucose levels in all patients, as IGT and DM may be unmasked during somatropin therapy. Doses of concurrent antihyperglycemic drugs in patients with DM may require adjustment. (5.4).
- Intracranial hypertension (IH): Exclude preexisting papilledema. IH may develop, but is usually reversible after discontinuation or dose reduction (5.5).
- Hypersensitivity: Serious hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention (5.6).
- Fluid retention (e.g., edema, arthralgia, carpal tunnel syndrome- especially in adults): Reduce dose as necessary if such signs develop (5.7).
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increase in those with known hypoadrenalism (5.8).
- Hypothyroidism: Monitor thyroid function periodically as it may first become evident or worsen after initiation of somatropin (5.9).
- Slipped capital femoral epiphysis (SCFE): Evaluate any child with onset of a limp or hip/knee pain for possible SCFE (5.10).
- Progression of preexisting scoliosis: Monitor any child with scoliosis for progression of the curve (5.11).
- Pancreatitis: Consider pancreatitis in patients with persistent severe abdominal pain (5.16)
ADVERSE REACTIONS
Common somatropin-related adverse reactions include injection site reactions. Additional common adverse reactions in adults include edema, arthralgias, and carpal tunnel syndrome (6.1, 6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Inhibition of 11 β-Hydroxysteroid Dehydrogenase Type 1: May require the initiation of glucocorticoid replacement therapy. Patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance doses (7.1, 7.2).
- Glucocorticoid replacement: Should be carefully adjusted (7.2).
- Cytochrome P450 - Metabolized Drugs: Monitor carefully if used with somatropin (7.3).
- Oral estrogen: Larger doses of somatropin may be required in women (7.4).
- Insulin and/or other hypoglycemic agents: May require adjustment (7.5).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
1.2 Adult Patients
2 DOSAGE AND ADMINISTRATION
2.1 Dosing for Pediatric Patients
2.2 Dosing for Adult Patients
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness
5.2 Prader-Willi Syndrome (PWS) in Children
5.3 Neoplasms
5.4 Glucose Intolerance and Diabetes Mellitus
5.5 Intracranial Hypertension
5.6 Severe Hypersensitivity
5.7 Fluid Retention
5.8 Hypoadrenalism
5.9 Hypothyroidism
5.10 Slipped Capital Femoral Epiphysis (SCFE) in Pediatric Patients
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
5.12 Otitis Media and Cardiovascular Disorders in Patients with Turner Syndrome
5.13 Osteodystrophy in Pediatric Patients with Chronic Kidney Disease
5.14 Lipoatrophy
5.15 Laboratory Tests
5.16 Pancreatitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 11 β-Hydroxysteroid Dehydrogenase Type 1 (11βHSD-1)
7.2 Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment
7.3 Cytochrome P450 (CYP450)-Metabolized Drugs
7.4 Oral Estrogen
7.5 Insulin and/or Oral/Injectable Hypoglycemic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Gender Effect
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pubertal Patients with Growth Hormone Deficiency (GHD)
14.2 Pediatric Patients with Growth Failure Secondary to Chronic Kidney Disease (CKD)
14.3 Pediatric Patients with Turner Syndrome (TS)
14.4 Pediatric Patient with Idiopathic Short Stature (ISS)
14.5 Adult Growth Hormone Deficiency
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
Growth Hormone Deficiency (GHD) - Nutropin AQ® is indicated for the treatment of pediatric patients who have growth failure due to inadequate secretion of endogenous growth hormone (GH).
Growth Failure Secondary to Chronic Kidney Disease (CKD) - Nutropin AQ is indicated for the treatment of growth failure associated with CKD up to the time of renal transplantation. Nutropin AQ therapy should be used in conjunction with optimal management of CKD.
Idiopathic Short Stature (ISS) - Nutropin AQ is indicated for the treatment of ISS, also called non-GHD short stature, defined by height SDS ≤ –2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range, in pediatric patients whose epiphyses are not closed and for whom diagnostic evaluation excludes other causes associated with short stature that should be observed or treated by other means.
1.2 Adult Patients
Nutropin AQ is indicated for the replacement of endogenous GH in adults with GHD who meet either of the following two criteria:
Adult Onset: Patients who have GHD, either alone or associated with multiple hormone deficiencies (hypopituitarism), as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma; or
Childhood Onset: Patients who were GH deficient during childhood as a result of congenital, genetic, acquired, or idiopathic causes.
Patients who were treated with somatropin for GHD in childhood and whose epiphyses are closed should be reevaluated before continuation of somatropin therapy at the reduced dose level recommended for GH deficient adults. According to current standards, confirmation of the diagnosis of adult GHD in both groups involves an appropriate GH provocative test with two exceptions: (1) patients with multiple pituitary hormone deficiencies due to organic disease; and (2) patients with congenital/genetic GHD.
-
2 DOSAGE AND ADMINISTRATION
For subcutaneous injection.
Therapy with Nutropin AQ should be supervised by a physician who is experienced in the diagnosis and management of pediatric patients with short stature associated with growth hormone deficiency (GHD), chronic kidney disease, Turner syndrome, idiopathic short stature, or adult patients with either childhood-onset or adult-onset GHD.
2.1 Dosing for Pediatric Patients
Nutropin AQ dosage and administration schedule should be individualized for each patient. Response to growth hormone (GH) therapy in pediatric patients tends to decrease with time. However, in pediatric patients failure to increase growth rate, particularly during the first year of therapy, suggests the need for close assessment of compliance and evaluation of other causes of growth failure, such as hypothyroidism, under-nutrition, advanced bone age and antibodies to recombinant human GH (rhGH).
Treatment with Nutropin AQ for short stature should be discontinued when the epiphyses are fused.
Pediatric Growth Hormone Deficiency (GHD)
A weekly dosage of up to 0.3 mg/kg of body weight divided into daily subcutaneous injection is recommended.
In pubertal patients, a weekly dosage of up to 0.7 mg/kg divided daily may be used.
Growth Failure Secondary to Chronic Kidney Disease (CKD)
A weekly dosage of up to 0.35 mg/kg of body weight divided into daily subcutaneous injection is recommended.
Nutropin AQ therapy may be continued up to the time of renal transplantation.
In order to optimize therapy for patients who require dialysis, the following guidelines for injection schedule are recommended:
- Hemodialysis patients should receive their injection at night just prior to going to sleep or at least 3 to 4 hours after their hemodialysis to prevent hematoma formation due to the heparin.
- Chronic Cycling Peritoneal Dialysis (CCPD) patients should receive their injection in the morning after they have completed dialysis.
- Chronic Ambulatory Peritoneal Dialysis (CAPD) patients should receive their injection in the evening at the time of the overnight exchange.
2.2 Dosing for Adult Patients
Adult Growth Hormone Deficiency (GHD)
Either of two approaches to Nutropin AQ dosing may be followed: a weight-based regimen or a non-weight-based regimen.
Weight based – Based on the dosing regimen used in the original adult GHD registration trials, the recommended dosage at the start of treatment is not more than 0.006 mg/kg daily. The dose may be increased according to individual patient requirements to a maximum of 0.025 mg/kg daily in patients ≤ 35 years and to a maximum of 0.0125 mg/kg daily in patients over 35 years old. Clinical response, side effects, and determination of age- and gender-adjusted serum insulin-like growth factor (IGF-1) concentrations should be used as guidance in dose titration.
Non-weight based – Alternatively, taking into account the published literature, a starting dose of approximately 0.2 mg/day (range, 0.15 to 0.30 mg/day) may be used without consideration of body weight. This dose can be increased gradually every 1 to 2 months by increments of approximately 0.1 to 0.2 mg/day, according to individual patient requirements based on the clinical response and serum IGF-1 concentrations. The dose should be decreased as necessary on the basis of adverse events and/or serum IGF-1 concentrations above the age- and gender-specific normal range.
Maintenance dosages vary considerably from person to person, and between male and female patients.
A lower starting dose and smaller dose increments should be considered for older patients, who are more prone to the adverse effects of somatropin than younger individuals. In addition, obese individuals are more likely to manifest adverse effects, when treated with a weight-based regimen. In order to reach the defined treatment goal, estrogen-replete women may need higher doses than men. Oral estrogen administration may increase the dose requirements in women.
2.3 Preparation and Administration
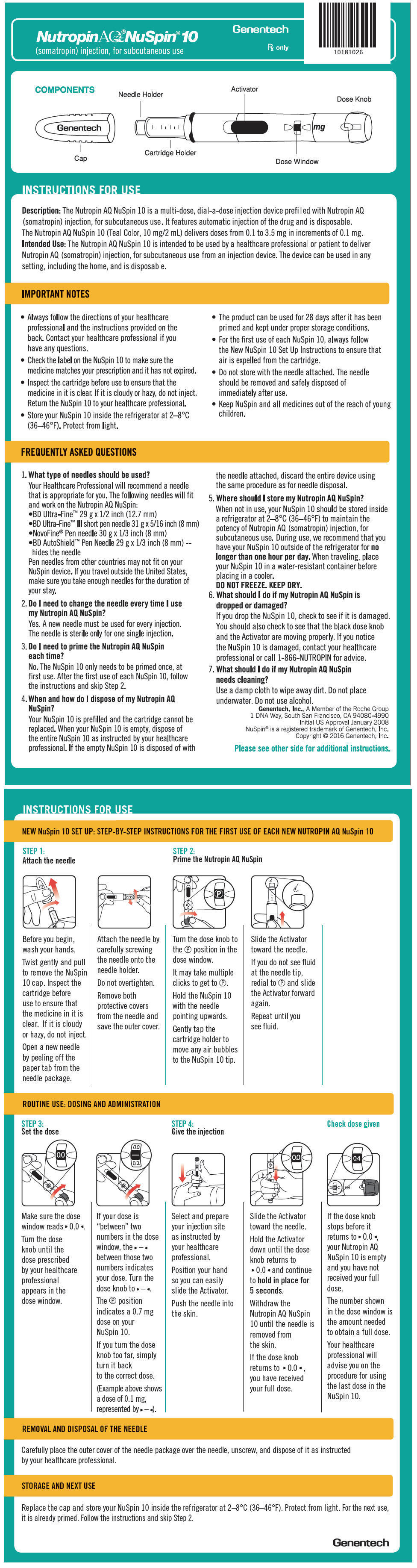
The solution should be clear immediately after removal from the refrigerator. Occasionally, after refrigeration, you may notice that small colorless particles of protein are present in the solution. This is not unusual for solutions containing proteins. Allow the pen cartridge or NuSpin® to come to room temperature and gently swirl. If the solution is cloudy, the contents MUST NOT be injected.
Parenteral drug products should always be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Injection sites, which may be located on the thigh, upper arm, abdomen or buttock, should always be rotated to avoid lipoatrophy.
Nutropin AQ Pen Cartridge
The Nutropin AQ Pen 10 and 20 mg Cartridges are color-banded to help ensure appropriate use with the Nutropin AQ Pen delivery device. Each cartridge must be used with its corresponding color-coded Nutropin AQ Pen [See Dosage Forms and Strengths (3)].
Wipe the septum of the Nutropin AQ Pen Cartridge with rubbing alcohol or an antiseptic solution to prevent contamination of the contents by microorganisms that may be introduced by repeated needle insertions. It is recommended that Nutropin AQ be administered using sterile, disposable needles. Follow the directions provided in the Nutropin AQ Pen Instructions for Use.
The Nutropin AQ Pen 10 allows for administration of a minimum dose of 0.1 mg to a maximum dose of 4.0 mg, in 0.1 mg increments.
The Nutropin AQ Pen 20 allows for administration of a minimum dose of 0.2 mg to a maximum dose of 8.0 mg, in 0.2 mg increments.
Nutropin AQ NuSpin
The Nutropin AQ NuSpin 5, 10 and 20 are multi-dose, dial-a-dose injection devices prefilled with Nutropin AQ in a 5 mg/2 mL, 10 mg/2 mL or 20 mg/2 mL cartridge, respectively, for subcutaneous use. It is recommended that Nutropin AQ be administered using sterile, disposable needles. Follow the directions provided in the Nutropin AQ NuSpin 5, 10 or 20 Instructions for Use.
The Nutropin AQ NuSpin 5 allows for administration of a minimum dose of 0.05 mg to a maximum dose of 1.75 mg, in increments of 0.05 mg.
The Nutropin AQ NuSpin 10 allows for administration of a minimum dose of 0.1 mg to a maximum dose of 3.5 mg, in increments of 0.1 mg.
The Nutropin AQ NuSpin 20 allows for administration of a minimum dose of 0.2 mg to a maximum dose of 7.0 mg, in increments of 0.2 mg.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Acute Critical Illness
Treatment with pharmacologic amounts of somatropin is contraindicated in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure. [see Warnings and Precautions (5.1)].
- Prader-Willi Syndrome (PWS) in Children
Somatropin is contraindicated in patients with PWS who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment. There have been reports of sudden death when somatropin was used in such patients. Nutropin AQ is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed PWS. [see Warnings and Precautions (5.2)].
- Active Malignancy
In general, somatropin is contraindicated in the presence of active malignancy. Any pre-existing malignancy should be inactive and its treatment complete prior to instituting therapy with somatropin. Somatropin should be discontinued if there is evidence of recurrent activity. Since growth hormone deficiency (GHD) may be an early sign of the presence of a pituitary tumor (or, rarely, other brain tumors), the presence of such tumors should be ruled out prior to initiation of treatment. Somatropin should not be used in patients with any evidence of progression or recurrence of an underlying intracranial tumor [see Warnings and Precautions (5.3)].
- Hypersensitivity
Nutropin AQ is contraindicated in patients with a known hypersensitivity to somatropin or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products [(see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness
Increased mortality in patients with acute critical illnesses due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic amounts of somatropin [see Contraindications (4)]. Two placebo-controlled clinical trials in non-GHD adult patients (n = 522) with these conditions in intensive care units revealed a significant increase in mortality (42% vs. 19%) among somatropin-treated patients (doses 5.3–8 mg/day) compared to those receiving placebo. The safety of continuing somatropin treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with somatropin in patients having acute critical illnesses should be weighed against the potential risk.
5.2 Prader-Willi Syndrome (PWS) in Children
There have been reports of fatalities after initiating therapy with somatropin in pediatric patients with PWS who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with PWS syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. If during treatment with somatropin, patients show signs of upper airway obstruction (including onset of or increased snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with PWS treated with somatropin should also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively [see Contraindications (4)]. Nutropin AQ is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed PWS.
5.3 Neoplasms
In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm has been reported. Intracranial tumors, in particular meningiomas, were the most common of these second neoplasms. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence [see Contraindications (4)]. Monitor all patients with a history of GHD secondary to an intracranial neoplasm routinely while on somatropin therapy for progression or recurrence of the tumor.
Because children with certain rare genetic causes of short stature have an increased risk of developing malignancies, practitioners should thoroughly consider the risks and benefits of starting somatropin in these patients. If treatment with somatropin is initiated, these patients should be carefully monitored for development of neoplasms.
Monitor patients on somatropin therapy carefully for increased growth, or potential malignant changes, of preexisting nevi.
5.4 Glucose Intolerance and Diabetes Mellitus
Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses in susceptible patients. As a result, previously undiagnosed impaired glucose tolerance (IGT) and overt diabetes mellitus may be unmasked during somatropin treatment, and new onset type 2 diabetes mellitus has been reported in patients taking somatropin. Therefore, glucose levels should be monitored periodically in all patients treated with somatropin, especially in those with risk factors for diabetes, such as obesity, Turner syndrome (TS), or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or IGT should be monitored closely during somatropin therapy. The doses of antihyperglycemic drugs (i.e. insulin or oral/injectable agents) may require adjustment when somatropin therapy is instituted in these patients.
5.5 Intracranial Hypertension
Intracranial Hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in a small number of patients treated with somatropin products. Symptoms usually occurred within the first eight (8) weeks after the initiation of somatropin therapy. In all reported cases, IH-associated signs and symptoms rapidly resolved after cessation of therapy or a reduction of the somatropin dose. Funduscopic examination should be performed routinely before initiating treatment with somatropin to exclude preexisting papilledema, and periodically during the course of somatropin therapy. If papilledema is observed by funduscopy during somatropin treatment, treatment should be stopped. If somatropin-induced IH is diagnosed, treatment with somatropin can be restarted at a lower dose after IH-associated signs and symptoms have resolved. Patients with TS, chronic kidney disease (CKD), and PWS may be at increased risk for the development of IH.
5.6 Severe Hypersensitivity
Serious systemic hypersensitivity reactions including anaphylactic reaction and angioedema have been reported with postmarketing use of somatropin products. Patients and caregivers should be informed that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs.
5.7 Fluid Retention
Fluid retention during somatropin replacement therapy in adults may occur. Clinical manifestations of fluid retention (e.g., edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) are usually transient and dose dependent.
5.8 Hypoadrenalism
Patients receiving somatropin therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of somatropin treatment [see Section 7.1, 11-β Hydroxysteroid Dehydrogenase Type 1].
5.9 Hypothyroidism
Undiagnosed/untreated hypothyroidism may prevent an optimal response to somatropin, in particular, the growth response in children. Patients with TS have an inherently increased risk of developing autoimmune thyroid disease and primary hypothyroidism. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Therefore, patients treated with somatropin should have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
5.10 Slipped Capital Femoral Epiphysis (SCFE) in Pediatric Patients
SCFE may occur more frequently in patients with endocrine disorders (including GHD and TS) or in patients undergoing rapid growth. Any pediatric patient with the onset of a limp or complaints of hip or knee pain during somatropin therapy should be carefully evaluated.
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
Progression of scoliosis can occur in patients who experience rapid growth. Because somatropin increases growth rate, patients with a history of scoliosis who are treated with somatropin should be monitored for progression of scoliosis. However, somatropin has not been shown to increase the occurrence of scoliosis. Skeletal abnormalities including scoliosis are commonly seen in untreated TS patients. Scoliosis is also commonly seen in untreated patients with PWS. Physicians should be alert to these abnormalities, which may manifest during somatropin therapy.
5.12 Otitis Media and Cardiovascular Disorders in Patients with Turner Syndrome
Patients with TS should be evaluated carefully for otitis media and other ear disorders, as these patients have an increased risk of ear and hearing disorders. Somatropin treatment may increase the occurrence of otitis media in patients with TS. In addition, patients with TS should be monitored closely for cardiovascular disorders (e.g., hypertension, aortic aneurysm or dissection, stroke) as these patients are also at increased risk for these conditions.
5.13 Osteodystrophy in Pediatric Patients with Chronic Kidney Disease
Children with growth failure secondary to CKD should be examined periodically for evidence of progression of renal osteodystrophy. SCFE or avascular necrosis of the femoral head may be seen in children with advanced renal osteodystrophy, and it is uncertain whether these problems are affected by somatropin therapy. X-rays of the hip should be obtained prior to initiating somatropin therapy in CKD patients and physicians and parents should be alert to the development of a limp or complaints of hip or knee pain in these patients treated with Nutropin AQ. No studies have been completed evaluating Nutropin AQ therapy in patients who have received renal transplants. Currently, treatment of patients with functioning renal allografts is not indicated.
5.14 Lipoatrophy
When somatropin is administered subcutaneously at the same site over a long period of time, tissue atrophy may result. This can be avoided by rotating the injection site [see Dosage and Administration (2.3)].
5.15 Laboratory Tests
Serum levels of inorganic phosphorus, alkaline phosphatase, and parathyroid hormone (PTH), and IGF-1 may increase during somatropin therapy.
5.16 Pancreatitis
Cases of pancreatitis have been reported rarely in children and adults receiving somatropin treatment, with some evidence supporting a greater risk in children compared with adults. Published literature indicates that girls who have TS may be at greater risk than other somatropin-treated children. Pancreatitis should be considered in any somatropin–treated patient, especially a child, who develops persistent severe abdominal pain.
-
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling:
- Increased mortality in patients with acute critical illness [see Warnings and Precautions (5.1)]
- Fatalities in children with Prader-Willi syndrome [see Warnings and Precautions (5.2)]
- Neoplasms in pediatric patients [see Warnings and Precautions (5.3)]
- Glucose intolerance and diabetes mellitus [see Warnings and Precautions (5.4)]
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- Severe hypersensitivity [see Warnings and Precautions (5.6)]
- Fluid retention [see Warnings and Precautions (5.7)]
- Hypoadrenalism [see Warnings and Precautions (5.8)]
- Hypothyroidism [see Warnings and Precautions (5.9)]
- Slipped capital femoral epiphysis in pediatric patients [see Warnings and Precautions (5.10)]
- Progression of preexisting scoliosis in pediatric patients [see Warnings and Precautions (5.11)]
- Otitis media and cardiovascular disorders in patients with Turner syndrome [see Warnings and Precautions (5.12)]
- Osteodystrophy in pediatric patients with chronic kidney disease [see Warnings and Precautions (5.13)]
- Lipoatrophy [see Warnings and Precautions (5.14)]
- Pancreatitis [see Warnings and Precautions (5.16)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed during the clinical trials performed with one somatropin formulation cannot always be directly compared to the rates observed during the clinical trials performed with a second somatropin formulation, and may not reflect the adverse reaction rates observed in practice.
Pediatric Patients
Growth Hormone Deficiency (GHD)
Injection site discomfort has been reported. This is more commonly observed in children switched from another somatropin product to Nutropin AQ.
Turner Syndrome
In a randomized, controlled trial, there was a statistically significant increase, as compared to untreated controls, in otitis media (43% vs. 26%) and ear disorders (18% vs. 5%) in patients receiving somatropin.
Idiopathic Short Stature (ISS)
In a post-marketing surveillance study, the National Cooperative Growth Study (NCGS), the pattern of adverse events in over 8,000 patients with ISS was consistent with the known safety profile of growth hormone (GH), and no new safety signals attributable to GH were identified. The frequency of protocol-defined targeted adverse events is described in the table, below.
Table 1 Protocol-Defined Targeted Adverse Events in the ISS NCGS Cohort Reported Events NCGS
(N = 8018)AVN = avascular necrosis; SCFE = slipped capital femoral epiphysis.
Data obtained with several rhGH products (Nutropin, Nutropin AQ, Nutropin Depot and Protropin).Any Adverse Event Overall 103 (1.3%) Targeted Adverse Event Overall 103 (1.3%) Injection-site reaction 28 (0.3%) New onset or progression of scoliosis 16 (0.2%) Gynecomastia 12 (0.1%) Any new onset or recurring tumor (benign) 12 (0.1%) Arthralgia or arthritis 10 (0.1%) Diabetes mellitus 5 (0.1%) Edema 5 (0.1%) Cancer, neoplasm (new onset or recurrence) 4 (0.0%) Fracture 4 (0.0%) Intracranial hypertension 4 (0.0%) Abnormal bone or other growth 3 (0.0%) Central nervous system tumor 2 (0.0%) New or recurrent SCFE or AVN 2 (0.0%) Carpal tunnel syndrome 1 (0.0%) In subjects treated in a long-term study of Nutropin for ISS, mean fasting and postprandial insulin levels increased, while mean fasting and postprandial glucose levels remained unchanged. Mean hemoglobin A1c (A1C) levels rose slightly from baseline as expected during adolescence; sporadic values outside normal limits occurred transiently.
Adult Patients
Growth Hormone Deficiency
In clinical studies with Nutropin AQ in GHD adults, edema or peripheral edema was reported in 41% of GH-treated patients and 25% of placebo-treated patients. In GHD adults, arthralgias and other joint disorders were reported in 27% of GH-treated patients and 15% of placebo-treated patients.
Nutropin therapy in adults with GHD of adult-onset was associated with an increase of median fasting insulin level in the Nutropin 0.0125 mg/kg/day group from 9.0 µU/mL at baseline to 13.0 µU/mL at Month 12 with a return to the baseline median level after a 3-week post-washout period of GH therapy. In the placebo group there was no change from 8.0 µU/mL at baseline to Month 12, and after the post-washout period, the median level was 9.0 µU/mL. The between-treatment group difference on the change from baseline to Month 12 in median fasting insulin level was significant, p < 0.0001. In childhood-onset subjects, there was an increase of median fasting insulin level in the Nutropin 0.025 mg/kg/day group from 11.0 µU/mL at baseline to 20.0 µU/mL at Month 12, in the Nutropin 0.0125 mg/kg/day group from 8.5 µU/mL to 11.0 µU/mL, and in the placebo group from 7.0 µU/mL to 8.0 µU/mL. The between-treatment group differences for these changes were significant, p = 0.0007.
In subjects with adult-onset GHD, there were no between-treatment group differences on change from baseline to Month 12 in mean A1C level, p = 0.08. In childhood-onset GHD, the mean A1C level increased in the Nutropin 0.025 mg/kg/day group from 5.2% at baseline to 5.5% at Month 12, and did not change in the Nutropin 0.0125 mg/kg/day group from 5.1% at baseline or in the placebo group from 5.3% at baseline. The between-treatment group differences were significant, p = 0.009.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Nutropin with the incidence of antibodies to other products may be misleading. In the case of GH, antibodies with binding capacities lower than 2 mg/L have not been associated with growth attenuation. In a very small number of patients treated with somatropin, when binding capacity was greater than 2 mg/L, interference with the growth response was observed.
In clinical studies of pediatric patients that were treated with Nutropin for the first time, 0/107 GHD patients, 0/125 CKD patients, 0/112 TS, and 0/117 ISS patients screened for antibody production developed antibodies with binding capacities ≥ 2 mg/L at six months. In a clinical study of patients that were treated with Nutropin AQ for the first time, 0/38 GHD patients screened for antibody production for up to 15 months developed antibodies with binding capacities ≥ 2 mg/L.
Additional short-term immunologic and renal function studies were carried out in a group of pediatric patients with CKD after approximately one year of treatment to detect other potential adverse effects of antibodies to GH. Testing included measurements of C1q, C3, C4, rheumatoid factor, creatinine, creatinine clearance, and blood urea nitrogen (BUN). No adverse effects of GH antibodies were noted.
6.3 Post-Marketing Experience
Because these adverse events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The adverse events reported during post-marketing surveillance do not differ from those listed/discussed above in Sections 6.1 and 6.2 in children and adults.
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products [see Warnings and Precautions (5.6)].
Leukemia has been reported in a small number of GHD children treated with somatropin, somatrem (methionylated rhGH) and GH of pituitary origin. It is uncertain whether these cases of leukemia are related to GH therapy, the pathology of GHD itself, or other associated treatments such as radiation therapy. On the basis of current evidence, experts have not been able to conclude that GH therapy per se was responsible for these cases of leukemia. The risk for children with GHD, CKD, or TS, if any, remains to be established [see Contraindications (4) and Warnings and Precautions (5.3)].
The following additional adverse reactions have been reported in GH-treated patients: gynecomastia (children), and pancreatitis [(Children and adults, see Warnings and Precautions (5.16)].
-
7 DRUG INTERACTIONS
7.1 11 β-Hydroxysteroid Dehydrogenase Type 1 (11βHSD-1)
The microsomal enzyme 11βHSD-1 is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. Growth hormone (GH) and somatropin inhibit 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Introduction of somatropin treatment may result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations. As a consequence, previously undiagnosed central (secondary) hypoadrenalism may be unmasked and glucocorticoid replacement may be required in patients treated with somatropin. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of somatropin treatment; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1.
7.2 Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment
Pharmacologic glucocorticoid therapy and supraphysiologic glucocorticoid treatment may attenuate the growth-promoting effects of somatropin in children. Therefore, glucocorticoid replacement therapy should be carefully adjusted in children with concomitant GH and glucocorticoid deficiency to avoid both hypoadrenalism and an inhibitory effect on growth.
The use of Nutropin AQ in patients with Chronic Kidney Disease (CKD) requiring glucocorticoid therapy has not been evaluated. Concomitant glucocorticoid therapy may inhibit the growth promoting effect of Nutropin AQ. Therefore, if glucocorticoid replacement is required for CKD, the glucocorticoid dose should be carefully adjusted to avoid an inhibitory effect on growth. In the clinical trials there was no evidence of drug interactions with Nutropin and commonly used drugs used in the management of CKD.
7.3 Cytochrome P450 (CYP450)-Metabolized Drugs
Limited published data indicate that somatropin treatment increases CYP450-mediated antipyrine clearance in man. These data suggest that somatropin administration may alter the clearance of compounds known to be metabolized by CYP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine). Careful monitoring is advisable when somatropin is administered in combination with other drugs known to be metabolized by CYP450 liver enzymes. However, formal drug interaction studies have not been conducted.
7.4 Oral Estrogen
Because oral estrogens may reduce insulin-like growth factor (IGF-1) response to somatropin treatment, girls and women receiving oral estrogen replacement may require greater somatropin dosages [see Dosage and Administration (2.2)].
7.5 Insulin and/or Oral/Injectable Hypoglycemic Agents
In patients with diabetes mellitus requiring drug therapy, the dose of insulin and/or oral/injectable hypoglycemic agents may require adjustment when somatropin therapy is initiated [see Warnings and Precautions (5.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with Nutropin AQ. It is also not known whether Nutropin AQ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nutropin AQ should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
There have been no studies conducted with Nutropin AQ in nursing mothers. It is not known whether Nutropin AQ is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Nutropin AQ is administered to a nursing mother.
8.5 Geriatric Use
Clinical studies of Nutropin AQ did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly patients may be more sensitive to the action of somatropin, and therefore may be more prone to develop adverse reactions. A lower starting dose and smaller dose increments should be considered for older patients [see Dosage and Administration (2.2)].
8.6 Hepatic Impairment
No studies have been conducted for Nutropin AQ in patients with hepatic impairment. [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Subjects with chronic renal failure tend to have decreased somatropin clearance compared to those with normal renal function [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Short Term
Short-term overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Furthermore, overdose with somatropin is likely to cause fluid retention.
Long Term
Long-term overdosage could result in signs and symptoms of gigantism and/or acromegaly consistent with the known effects of excess growth hormone (GH) [see Dosage and Administration (2.2)].
-
11 DESCRIPTION
Nutropin AQ (somatropin) injection, for subcutaneous use is a human growth hormone (hGH) produced by recombinant DNA technology. Nutropin AQ has 191 amino acid residues and a molecular weight of 22,125 daltons. The amino acid sequence of the product is identical to that of pituitary-derived hGH. Nutropin AQ may contain not more than fifteen percent deamidated GH at expiration. The deamidated form of GH has been extensively characterized and has been shown to be safe and fully active.
Nutropin AQ is a sterile liquid intended for subcutaneous administration. The product is nearly isotonic at a concentration of 5 mg of GH per mL and has a pH of approximately 6.0.
Each pen cartridge or NuSpin contain either 5 mg, 10 mg or 20 mg of somatropin formulated in 17.4 mg sodium chloride, 5 mg phenol, 4 mg polysorbate 20, and 10 mM sodium citrate [see How Supplied/Storage and Handling (16)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Somatropin (as well as endogenous growth hormone) binds to dimeric growth hormone receptors located within the cell membranes of target tissue cells resulting in intracellular signal transduction and a host of pharmacodynamic effects. Some of these pharmacodynamic effects are primarily mediated by insulin-like growth factor (IGF-1) produced in the liver and also locally (e.g., skeletal growth, protein synthesis), while others are primarily a consequence of the direct effects of somatropin (e.g., lipolysis) [see Clinical Pharmacology (12.2)].
12.2 Pharmacodynamics
In vitro and in vivo preclinical and clinical testing have demonstrated that Nutropin AQ is therapeutically equivalent to pituitary-derived hGH. Pediatric patients who lack adequate endogenous growth hormone (GH) secretion, patients with chronic kidney disease (CKD), and patients with Turner syndrome (TS) that were treated with Nutropin AQ or Nutropin resulted in an increase in growth rate and an increase in IGF-1 levels similar to that seen with pituitary-derived hGH.
Tissue Growth
- A)
- Skeletal Growth: Nutropin AQ stimulates skeletal growth in pediatric patients with growth failure due to a lack of adequate secretion of endogenous GH or secondary to CKD and in patients with TS. Skeletal growth is accomplished at the epiphyseal plates at the ends of a growing bone. Growth and metabolism of epiphyseal plate cells are directly stimulated by GH and one of its mediators, IGF-I. Serum levels of IGF-I are low in children and adolescents who are GHD, but increase during treatment with somatropin. In pediatric patients, new bone is formed at the epiphyses in response to GH and IGF-I. This results in linear growth until these growth plates fuse at the end of puberty.
- B)
- Cell Growth: Treatment with somatropin results in an increase in both the number and the size of skeletal muscle cells.
- C)
- Organ Growth: GH influences the size of internal organs, including kidneys, and increases red cell mass. Treatment of hypophysectomized or genetic dwarf rats with somatropin results in organ growth that is proportional to the overall body growth. In normal rats subjected to nephrectomy-induced uremia, somatropin promoted skeletal and body growth.
Protein Metabolism
Linear growth is facilitated in part by GH-stimulated protein synthesis. This is reflected by nitrogen retention as demonstrated by a decline in urinary nitrogen excretion and blood urea nitrogen (BUN) during somatropin therapy.
Carbohydrate Metabolism
GH is a modulator of carbohydrate metabolism. For example, patients with inadequate secretion of GH sometimes experience fasting hypoglycemia that is improved by treatment with Nutropin AQ. Somatropin therapy may decrease insulin sensitivity. Untreated patients with CKD and TS have an increased incidence of glucose intolerance. Administration of somatropin to adults or children resulted in increases in serum fasting and postprandial insulin levels, more commonly in overweight or obese individuals. In addition, mean fasting and postprandial glucose and hemoglobin A1C levels remained in the normal range.
Lipid Metabolism
In GHD patients, administration of somatropin resulted in lipid mobilization, reduction in body fat stores, increased plasma fatty acids, and decreased plasma cholesterol levels.
Mineral Metabolism
The retention of total body potassium in response to somatropin administration apparently results from cellular growth. Serum levels of inorganic phosphorus may increase slightly in patients with inadequate secretion of endogenous GH, CKD, or TS during Nutropin AQ therapy due to metabolic activity associated with bone growth as well as increased tubular reabsorption of phosphate by the kidney. Serum calcium is not significantly altered in these patients. Sodium retention also occurs. Adults with childhood-onset GHD show low bone mineral density (BMD). Nutropin AQ therapy results in increases in serum alkaline phosphatase [see Warnings and Precautions (5.14)].
12.3 Pharmacokinetics
Absorption
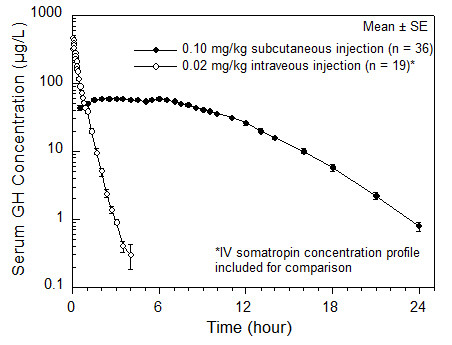
The absolute bioavailability of somatropin after subcutaneous administration in healthy adult males has been determined to be 81 ± 20%. The mean terminal t1/2 after subcutaneous administration is significantly longer than that seen after intravenous administration (2.1 ± 0.43 hours vs. 19.5 ± 3.1 minutes) indicating that the subcutaneous absorption of the compound is slow and rate-limiting.
Distribution
Animal studies with somatropin showed that GH localizes to highly perfused organs, particularly the liver and kidney. The volume of distribution at steady state for somatropin in healthy adult males is about 50 mL/kg body weight, approximating the serum volume.
Metabolism
Both the liver and kidney have been shown to be important metabolizing organs for GH. Animal studies suggest that the kidney is the dominant organ of clearance. GH is filtered at the glomerulus and reabsorbed in the proximal tubules. It is then cleaved within renal cells into its constituent amino acids, which return to the systemic circulation.
Elimination
The mean terminal t1/2 after intravenous administration of somatropin in healthy adult males is estimated to be 19.5 ± 3.1 minutes. Clearance of rhGH after intravenous administration in healthy adults and children is reported to be in the range of 116–174 mL/hr/kg.
Bioequivalence of Formulations
Nutropin AQ has been determined to be bioequivalent to Nutropin based on the statistical evaluation of area under the curve (AUC) and maximum concentration (Cmax).
Special Populations
Pediatric: Available literature data suggests that somatropin clearances are similar in adults and children.
Geriatrics: Limited published data suggest that the plasma clearance and average steady-state plasma concentration of somatropin may not be different between young and elderly patients.
Race: Reported values for half-lives for endogenous GH in normal adult black males are not different from observed values for normal adult white males. No data for other races are available.
Growth Hormone Deficiency: Reported values for clearance of somatropin in adults and children with GHD range 138–245 mL/hr/kg and are similar to those observed in healthy adults and children. Mean terminal t1/2 values following intravenous and subcutaneous administration in adult and pediatric GHD patients are also similar to those observed in healthy adult males.
Chronic Kidney Disease: Children and adults with CKD and end-stage renal disease (ESRD) tend to have decreased clearance compared to normals. In a study with six pediatric patients 7 to 11 years of age, the clearance of Nutropin was reduced by 21.5% and 22.6% after the intravenous infusion and subcutaneous injection, respectively, of 0.05 mg/kg of Nutropin compared to normal healthy adults. Endogenous GH production may also increase in some individuals with ESRD. However, no somatropin accumulation has been reported in children with CKD or ESRD dosed with current regimens.
Turner Syndrome: No pharmacokinetic data are available for exogenously administered somatropin. However, reported half-lives, absorption, and elimination rates for endogenous GH in this population are similar to the ranges observed for normal subjects and GHD populations.
Hepatic Insufficiency: A reduction in somatropin clearance has been noted in patients with severe liver dysfunction. The clinical significance of this decrease is unknown.
Gender: No gender-specific pharmacokinetic studies have been done with Nutropin AQ. The available literature indicates that the pharmacokinetics of somatropin are similar in men and women.
Table 2 Summary of Nutropin AQ Pharmacokinetic Parameters in Healthy Adult Males 0.1 mg (approximately 0.3 IU*)/kg SC Cmax
(µg/L)Tmax
(hr)t1/2
(hr)AUC0-∞
(μg ∙ hr/L)CL/Fsc
(mL/[hr ∙ kg])Abbreviations: AUC0-∞ = area under the curve, Cmax = maximum concentration, CL/Fsc = systemic clearance, CV% = coefficient of variation in %; SC = subcutaneous, Fsc = subcutaneous bioavailability (not determined), t1/2 = half-life. MEAN† 71.1 3.9 2.3 677 150 CV% 17 56 18 13 13 Figure 1
Single Dose Mean Growth Hormone Concentrations in Healthy Adult Males
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Pubertal Patients with Growth Hormone Deficiency (GHD)
One open label, multicenter, randomized clinical trial of two dosages of Nutropin was performed in pubertal patients with GHD. Ninety-seven patients (mean age 13.9 years, 83 male, 14 female) currently being treated with approximately 0.3 mg/kg/wk of GH were randomized to 0.3 mg/kg/wk or 0.7 mg/kg/wk Nutropin doses. All patients were already in puberty (Tanner stage ≥ 2) and had bone ages ≤ 14 years in males or ≤ 12 years in females. Mean baseline height standard deviation score (SDS) was –1.3.
The mean last measured height in all 97 patients after a mean duration of 2.7 ± 1.2 years, by analysis of covariance (ANCOVA) adjusting for baseline height, is shown below.
Table 3 Last Measured Height* by Sex and Nutropin Dose for Pubertal Patients with GHD Last Measured Height* (cm) Height Difference Between Groups (cm) Age (yr) 0.3 mg/kg/wk 0.7 mg/kg/wk Mean ± SD
(range)Mean ± SD Mean ± SD Mean ± SE - *
- Adjusted for baseline height
Male 17.2 ± 1.3
(13.6 to 19.4)170.9 ± 7.9
(n = 42)174.5 ± 7.9
(n = 41)3.6 ± 1.7 Female 15.8 ± 1.8
(11.9 to 19.3)154.7 ± 6.3
(n = 7)157.6 ± 6.3
(n = 7)2.9 ± 3.4 The mean height SDS at last measured height (n = 97) was –0.7 ± 1.0 in the 0.3 mg/kg/wk group and – 0.1 ± 1.2 in the 0.7 mg/kg/wk group. For patients completing 3.5 or more years (mean 4.1 years) of Nutropin treatment (15/49 patients in the 0.3 mg/kg/wk group and 16/48 patients in the 0.7 mg/kg/wk group), the mean last measured height was 166.1 ± 8.0 cm in the 0.3 mg/kg/wk group and 171.8 ± 7.1 cm in the 0.7 mg/kg/wk group, adjusting for baseline height and sex.
The mean change in bone age was approximately one year for each year in the study in both dose groups. Patients with baseline height SDS above –1.0 were able to attain normal adult heights with the 0.3 mg/kg/wk dose of Nutropin (mean height SDS at near-adult height = –0.1, n = 15).
Thirty-one patients had bone mineral density (BMD) determined by dual energy x-ray absorptiometry (DEXA) scans at study conclusion. The two dose groups did not differ significantly in mean SDS for total body BMD (–0.9 ± 1.9 in the 0.3 mg/kg/wk group vs. –0.8 ± 1.2 in the 0.7 mg/kg/wk group, n = 20) or lumbar spine BMD (–1.0 ± 1.0 in the 0.3 mg/kg/wk group vs. –0.2 ± 1.7 in the 0.7 mg/kg/wk group, n = 21).
Over a mean duration of 2.7 years, patients in the 0.7 mg/kg/wk group were more likely to have IGF-I values above the normal range than patients in the 0.3 mg/kg/wk group (27.7% vs. 9.0% of IGF-I measurements for individual patients). The clinical significance of elevated IGF-I values is unknown.
14.2 Pediatric Patients with Growth Failure Secondary to Chronic Kidney Disease (CKD)
Two multicenter, randomized, controlled clinical trials were conducted to determine whether treatment with Nutropin prior to renal transplantation in patients with CKD could improve their growth rates and height deficits. One study was a double-blind, placebo-controlled trial and the other was an open-label, randomized trial. The dose of Nutropin in both controlled studies was 0.05 mg/kg/day (0.35 mg/kg/week) administered daily by subcutaneous injection. Combining the data from those patients completing two years in the two controlled studies results in 62 patients treated with Nutropin and 28 patients in the control groups (either placebo-treated or untreated). The mean first year growth rate was 10.8 cm/yr for Nutropin-treated patients, compared with a mean growth rate of 6.5 cm/yr for placebo/untreated controls (p < 0.00005). The mean second year growth rate was 7.8 cm/yr for the Nutropin-treated group, compared with 5.5 cm/yr for controls (p < 0.00005). There was a significant increase in mean height SDS in the Nutropin group (–2.9 at baseline to –1.5 at Month 24, n = 62) but no significant change in the controls (-2.8 at baseline to –2.9 at Month 24, n = 28). The mean third year growth rate of 7.6 cm/yr in the Nutropin-treated patients (n = 27) suggests that Nutropin stimulates growth beyond two years. However, there are no control data for the third year because control patients crossed over to Nutropin treatment after two years of participation. The gains in height were accompanied by appropriate advancement of skeletal age. These data demonstrate that Nutropin therapy improves growth rate and corrects the acquired height deficit associated with CKD.
The North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) has reported data for growth post-transplant in children who did not receive GH prior to transplantation as well as children who did receive Nutropin during the clinical trials prior to transplantation. The average change in height SDS during the initial two years post-transplant was 0.15 for the 2,391 patients who did not receive GH pre-transplant and 0.28 for the 57 patients who did1. For patients who were followed for 5 years post-transplant, the corresponding changes in height SDS were also similar between groups.
14.3 Pediatric Patients with Turner Syndrome (TS)
Three US studies, two long-term, open-label, multicenter, historically controlled studies (Studies 1 and 2), and one long-term, randomized, dose-response study (Study 3) and one Canadian, long-term, randomized, open-label, multicenter, concurrently controlled study, were conducted to evaluate the efficacy of somatropin treatment of short stature due to TS.
In the US Studies 1 and 2, the effect of long-term GH treatment (0.375 mg/kg/week given either 3 times per week or daily) on adult height was determined by comparing adult heights in the treated patients with those of age-matched historical controls with TS who received no growth-promoting therapy. In Study 1, estrogen treatment was delayed until patients were at least age 14. GH therapy resulted in a mean adult height gain of 7.4 cm (mean duration of GH therapy of 7.6 years) vs. matched historical controls by ANCOVA.
In Study 2, patients treated with early Nutropin therapy (before 11 years of age) were randomized to receive estrogen-replacement therapy (conjugated estrogens, 0.3 mg escalating to 0.625 mg daily) at either age 12 or 15 years. Compared with matched historical controls, early Nutropin therapy (mean duration of 5.6 years) combined with estrogen replacement at age 12 years resulted in an adult height gain of 5.9 cm (n = 26), whereas girls who initiated estrogen at age 15 years (mean duration of Nutropin therapy 6.1 years) had a mean adult height gain of 8.3 cm (n = 29). Patients who initiated Nutropin after age 11 (mean age 12.7 years; mean duration of Nutropin therapy 3.8 years) had a mean adult height gain of 5.0 cm (n = 51).
Thus, in Studies 1 and 2, the greatest improvement in adult height was observed in patients who received early GH treatment and estrogen after age 14 years.
In Study 3, a randomized, blinded dose-response study, patients were treated from a mean age of 11.1 years for a mean duration of 5.3 years with a weekly GH dose of either 0.27 mg/kg or 0.36 mg/kg administered in divided doses 3 or 6 times weekly. The mean near-final height of GH-treated patients was 148.7 ± 6.5 cm (n = 31). When compared to historical control data, the mean gain in adult height was approximately 5 cm.
The Canadian randomized study compared near-adult height outcomes for GH-treated patients to those of a concurrent control group who received no injections. The somatropin-treated patients received a dosage of 0.3 mg/kg/week given in divided doses 6 times per week from a mean age of 11.7 years for a mean duration of 4.7 years. Puberty was induced with a standardized estrogen regimen initiated at 13 years of age for both treatment groups. The somatropin-treated group (n = 27) attained a mean (± SD) near final height of 146.0 ± 6.2 cm; the untreated control group (n = 19) attained a near final height of 142.1 ± 4.8 cm. By ANCOVA (with adjustments for baseline height and mid-parental height), the effect of GH-treatment was a mean height increase of 5.4 cm (p = 0.001).
In summary, patients with TS (total n = 181 from the 4 studies above) treated to adult height achieved statistically significant average height gains ranging from 5.0–8.3 cm.
Table 4 Summary of Efficacy Results in Turner Syndrome * Study Group Study Design† N at Adult Height GH Age (yr) Estrogen Age (yr) GH Duration (yr) Adult Height Gain (cm)‡ - *
- Data shown are mean values.
- †
- RCT: randomized controlled trial; MHT: matched historical controlled trial;
RDT: randomized dose-response trial. - ‡
- Analysis of covariance vs. controls.
- §
- A = GH age < 11 yr, estrogen age 15 yr.
B = GH age < 11 yr, estrogen age 12 yr.
C = GH age > 11 yr, estrogen at Month 12. - ¶
- Compared with historical data.
US 1 MHT 17 9.1 15.2 7.6 7.4 US 2 A§ MHT 29 9.4 15.0 6.1 8.3 B§ 26 9.6 12.3 5.6 5.9 C§ 51 12.7 13.7 3.8 5.0 US 3 RDT 31 11.1 8–13.5 5.3 ~5¶ Canadian RCT 27 11.7 13 4.7 5.4 14.4 Pediatric Patient with Idiopathic Short Stature (ISS)
A long-term, open-label, multicenter study was conducted to examine the safety and efficacy of Nutropin in pediatric patients with ISS, also called non-growth hormone deficient short stature. For the first year, 122 pre-pubertal subjects over the age of 5 years with stimulated serum GH ≥ 10 ng/mL were randomized into two treatment groups of approximately equal size; one group was treated with Nutropin 0.3 mg/kg weekly divided into three doses per week and the other group served as untreated controls. For the second and subsequent years of the study, all subjects were re-randomized to receive the same total weekly dose of Nutropin (0.3 mg/kg weekly) administered either daily or three times weekly. Treatment with Nutropin was continued until a subject's bone age was > 15.0 years (boys) or > 14.0 years (girls) and the growth rate was < 2 cm/yr, after which subjects were followed until adult height was achieved. The mean baseline values were: height SDS–2.8, IGF-I SDS -0.9, age 9.4 years, bone age 7.8 years, growth rate 4.4 cm/yr, mid-parental target height SDS –0.7, and Bayley-Pinneau predicted adult height SDS –2.3. Nearly all subjects had predicted adult height that was less than mid-parental target height.
During the one-year controlled phase of the study, the mean height velocity increased by 0.5 ± 1.8 cm (mean ± SD) in the no-treatment control group and by 3.1 ± 1.7 cm in the Nutropin group (p < 0.0001). For the same period of treatment the mean height SDS increased by 0.4 ± 0.2 and remained unchanged (0.0 ± 0.2) in the control group (p < 0.001).
Of the 118 subjects who were treated with Nutropin (70%) reached near-adult height (hereafter called adult height) after 2–10 years of Nutropin therapy. Their last measured height, including post-treatment follow-up, was obtained at a mean age of 18.3 years in males and 17.3 years in females. The mean duration of therapy was 6.2 and 5.5 years, respectively. Adult height was greater than pretreatment predicted adult height in 49 of 60 males (82%) and 19 of 23 females (83%). The mean difference between adult height and pretreatment predicted adult height was 5.2 cm (2.0 inches) in males and 6.0 cm (2.4 inches) in females (p < 0.0001 for both). The table (below) summarizes the efficacy data.
Table 5 Long-Term Efficacy in ISS (Mean ± SD) Characteristic Males (n = 60) Females (n = 23) - *
- p < 0.0001 versus zero.
Adult height (cm) 166.3 ± 5.8 153.1 ± 4.8 Pretreatment predicted adult height (cm) 161.1 ± 5.5 147.1 ± 5.1 Adult height minus pretreatment predicted adult height (cm) + 5.2 ± 5.0* + 6.0 ± 5.0* Adult height SDS –1.5 ± 0.8 –1.6 ± 0.7 Pretreatment predicted adult height SDS –2.2 ± 0.8 –2.5 ± 0.8 Adult height minus pretreatment predicted adult height SDS + 0.7 ± 0.7* + 0.9 ± 0.8* Nutropin therapy resulted in an increase in mean IGF-I SDS from –0.9 ± 1.0 to –0.2 ± 0.9 in Treatment Year 1. During continued treatment, mean IGF-I levels remained close to the normal mean. IGF-I SDS above + 2 occurred sporadically in 14 subjects.
14.5 Adult Growth Hormone Deficiency
Two multicenter, double-blind, placebo-controlled clinical trials were conducted in growth hormone-deficient adults. Study 1 was conducted in subjects with adult-onset GHD (n = 166), mean age 48.3 years, at doses of 0.0125 or 0.00625 mg/kg/day; doses of 0.025 mg/kg/day were not tolerated in these subjects. Study 2 was conducted in previously treated subjects with childhood-onset GHD (n = 64), mean age 23.8 years, at randomly assigned doses of 0.025 or 0.0125 mg/kg/day. The studies were designed to assess the effects of replacement therapy with Nutropin on body composition.
Significant changes from baseline to Month 12 of treatment in body composition (i.e., total body % fat mass, trunk % fat mass, and total body % lean mass by DEXA scan) were seen in all Nutropin groups in both studies (p < 0.0001 for change from baseline and vs. placebo), whereas no statistically significant changes were seen in either of the placebo groups. In the adult-onset study, the Nutropin group improved mean total body fat from 35.0% to 31.5%, mean trunk fat from 33.9% to 29.5%, and mean lean body mass from 62.2% to 65.7%, whereas the placebo group had mean changes of 0.2% or less (p = not significant). Due to the possible effect of GH-induced fluid retention on DEXA measurements of lean body mass, DEXA scans were repeated approximately 3 weeks after completion of therapy; mean % lean body mass in the Nutropin group was 65.0%, a change of 2.8% from baseline, compared with a change of 0.4% in the placebo group (p < 0.0001 between groups).
In the childhood-onset study, the high-dose Nutropin group improved mean total body fat from 38.4% to 32.1%, mean trunk fat from 36.7% to 29.0%, and mean lean body mass from 59.1% to 65.5%; the low-dose Nutropin group improved mean total body fat from 37.1% to 31.3%, mean trunk fat from 37.9% to 30.6%, and mean lean body mass from 60.0% to 66.0%; the placebo group had mean changes of 0.6% or less (p = not significant).
Table 6 Mean Changes from Baseline to Month 12 in Proportion of Fat and Lean by DEXA for Adult- and Childhood- Onset GHD Studies Adult Onset
(Study 1)Childhood Onset
(Study 2)Proportion Placebo
(n = 62)Nutropin
(n = 63)Between-Groups t-test p-value Placebo
(n = 13)Nutropin 0.0125 mg/kg/day
(n = 15)Nutropin 0.025 mg/kg/day
(n = 15)Placebo vs. Pooled Nutropin t-test p-value NA = not available Total body percent fat Baseline 36.8 35.0 0.38 35.0 37.1 38.4 0.45 Month 12 36.8 31.5 — 35.2 31.3 32.1 — Baseline to Month 12 change -0.1 -3.6 < 0.0001 + 0.2 -5.8 -6.3 < 0.0001 Post-washout 36.4 32.2 — NA NA NA — Baseline to post-washout change -0.4 -2.8 < 0.0001 NA NA NA — Trunk percent fat Baseline 35.3 33.9 0.50 32.5 37.9 36.7 0.23 Month 12 35.4 29.5 — 33.1 30.6 29.0 — Baseline to Month 12 change 0.0 -4.3 < 0.0001 + 0.6 -7.3 -7.6 < 0.0001 Post-washout 34.9 30.5 — NA NA NA — Baseline to post-washout change -0.3 -3.4 — NA NA NA — Total body percent lean Baseline 60.4 62.2 0.37 62.0 60.0 59.1 0.48 Month 12 60.5 65.7 — 61.8 66.0 65.5 — Baseline to Month 12 change + 0.2 + 3.6 < 0.0001 -0.2 + 6.0 + 6.4 < 0.0001 Post-washout 60.9 65.0 — NA NA NA — Baseline to post-washout change + 0.4 + 2.8 < 0.0001 NA NA NA — In the adult-onset study, significant decreases from baseline to Month 12 in low-density lipoprotein (LDL) cholesterol and LDL:high-density lipoprotein (HDL) ratio were seen in the Nutropin group compared to the placebo group, p < 0.02; there were no statistically significant between-group differences in change from baseline to Month 12 in total cholesterol, HDL cholesterol, or triglycerides. In the childhood-onset study significant decreases from baseline to Month 12 in total cholesterol, LDL cholesterol, and LDL:HDL ratio were seen in the high-dose Nutropin group only, compared to the placebo group, p < 0.05. There were no statistically significant between-group differences in HDL cholesterol or triglycerides from baseline to Month 12.
In the childhood-onset study, 55% of the patients had decreased spine BMD (z-score < –1) at baseline. The administration of Nutropin (n = 16) (0.025 mg/kg/day) for two years resulted in increased spine BMD from baseline when compared to placebo (n = 13) (4.6% vs. 1.0%, respectively, p < 0.03); a transient decrease in spine BMD was seen at six months in the Nutropin-treated patients. Thirty-five percent of subjects treated with this dose had supraphysiological levels of IGF-I at some point during the study, which may carry unknown risks. No significant improvement in total body BMD was found when compared to placebo. A lower GH dose (0.0125 mg/kg/day) did not show significant increments in either of these bone parameters when compared to placebo. No statistically significant effects on BMD were seen in the adult-onset study where patients received GH (0.0125 mg/kg/day) for one year.
Muscle strength, physical endurance, and quality of life measurements were not markedly abnormal at baseline, and no statistically significant effects of Nutropin therapy were observed in the two studies.
A subsequent 32-week, multicenter, open-label, controlled clinical trial was conducted using Nutropin AQ, Nutropin Depot, or no treatment in adults with both adult-onset and childhood-onset GHD. Subjects were randomized into the three groups to evaluate effects on body composition, including change in visceral adipose tissue (VAT) as determined by computed tomography (CT) scan.
For subjects evaluable for change in VAT in the Nutropin AQ (n = 44) and untreated (n = 19) groups, the mean age was 46.2 years and 78% had adult-onset GHD. Subjects in the Nutropin AQ group were treated at doses up to 0.012 mg/kg per day in women (all of whom received estrogen replacement therapy) and men under age 35 years, and up to 0.006 mg/kg per day in men over age 35 years.
The mean absolute change in VAT from baseline to Week 32 was –10.7 cm2 in the Nutropin AQ group and + 8.4 cm2 in the untreated group (p = 0.013 between groups). There was a 6.7% VAT loss in the Nutropin AQ group (mean percent change from baseline to Week 32) compared with a 7.5% increase in the untreated group (p = 0.012 between groups). The effect of reducing VAT in adult GHD patients with Nutropin AQ on long-term cardiovascular morbidity and mortality has not been determined.
Table 7 Visceral Adipose Tissue by Computed Tomography Scan: Percent Change and Absolute Change from Baseline to Week 32 in Study 3 Nutropin AQ
(n = 44)Untreated
(n = 19)Treatment Difference (adjusted mean) p-value VAT = visceral adipose tissue. - *
- ANCOVA using baseline VAT as a covariate
Baseline VAT (cm2) (mean) 126.2 123.3 Change in VAT (cm2) (adjusted mean) – 10.7 + 8.4 – 19.1 0.013* Percent change in VAT (adjusted mean) – 6.7 + 7.5 – 14.2 0.012* -
16 HOW SUPPLIED/STORAGE AND HANDLING
Pen Cartridge (2 mL): 10 mg
20 mgNDC 50242-043-14
NDC 50242-073-01Nutropin AQ NuSpin (2 mL): 5 mg
10 mg
20 mgNDC 50242-075-01
NDC 50242-074-01
NDC 50242-076-01Storage and Handling
Nutropin AQ cartridge and NuSpin injection device contents are stable for 28 days after initial use when stored at 2–8°C/36–46°F (under refrigeration). Avoid freezing Nutropin AQ in the cartridge or NuSpin injection device. Nutropin AQ is light sensitive and the cartridges and Nutropin AQ NuSpin should be protected from light. Store the cartridge and Nutropin AQ NuSpin injection device refrigerated in a dark place when they are not in use.
-
17 PATIENT COUNSELING INFORMATION
Patients being treated with Nutropin AQ (and/or their parents) should be informed about the potential benefits and risks associated with Nutropin AQ treatment, including a review of the contents of the INSTRUCTIONS FOR USE. This information is intended to better educate patients (and caregivers); it is not a disclosure of all possible adverse or intended effects.
Patients and caregivers who will administer Nutropin AQ should receive appropriate training and instruction on the proper use of Nutropin AQ from the physician or other suitably qualified health care professional. A puncture-resistant container for the disposal of used syringes and needles should be strongly recommended. Patients and/or parents should be thoroughly instructed in the importance of proper disposal, and cautioned against any reuse of needles and syringes. This information is intended to aid in the safe and effective administration of the medication.
Please see the accompanying directions for use of the delivery device.
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
PRINCIPAL DISPLAY PANEL - 5 mg NuSpin Carton
5 mg
NutropinAQ®NuSpin® 5
(somatropin) injection, for subcutaneous use
5 mg/2 mL (2.5 mg/mL)NDC 50242-075-01
Contents: One Nutropin AQ® NuSpin® 5, Instructions for Use, and Package Insert. Each Nutropin AQ® NuSpin® 5 contains 5 mg
(approximately 15 IU) of Nutropin AQ® (somatropin) injection, for subcutaneous use, formulated in 17.4 mg sodium chloride, 5 mg
phenol, 4 mg polysorbate 20, and 10 mM sodium citrate in 2 mL (2.5 mg/mL).
Usage and Administration: For subcutaneous use. Your healthcare professional will recommend a needle that is appropriate
for you (needles not included). See enclosed Package Insert and Instructions for Use.
Storage: Refrigerate at 2–8°C (36–46°F). DO NOT FREEZE. PROTECT FROM LIGHT.Rx only
KEEP REFRIGERATED
Genentech
10181028


-
PRINCIPAL DISPLAY PANEL - 10 mg NuSpin Carton
10 mg
NutropinAQ®NuSpin® 10
(somatropin) injection, for subcutaneous use
10 mg/2 mL (5 mg/mL)NDC 50242-074-01
Contents: One Nutropin AQ® NuSpin® 10, Instructions for Use, and Package Insert. Each Nutropin AQ® NuSpin® 10 contains 10 mg
(approximately 30 IU) of Nutropin AQ® (somatropin) injection, for subcutaneous use, formulated in 17.4 mg sodium chloride, 5 mg
phenol, 4 mg polysorbate 20, and 10 mM sodium citrate in 2 mL (5 mg/mL).
Usage and Administration: For subcutaneous use. Your healthcare professional will recommend a needle that is appropriate
for you (needles not included). See enclosed Package Insert and Instructions for Use.
Storage: Refrigerate at 2–8°C (36–46°F). DO NOT FREEZE. PROTECT FROM LIGHT.Rx only
KEEP REFRIGERATED
Genentech
10181029


-
PRINCIPAL DISPLAY PANEL - 20 mg NuSpin Carton
20 mg
NutropinAQ®NuSpin® 20
(somatropin) injection, for subcutaneous use
20 mg/2 mL (10 mg/mL)NDC 50242-076-01
Contents: One Nutropin AQ® NuSpin® 20, Instructions for Use, and Package Insert. Each Nutropin AQ® NuSpin® 20 contains 20 mg
(approximately 60 IU) of Nutropin AQ® (somatropin) injection, for subcutaneous use, formulated in 17.4 mg sodium chloride, 5 mg
phenol, 4 mg polysorbate 20, and 10 mM sodium citrate in 2 mL (10 mg/mL).
Usage and Administration: For subcutaneous use. Your healthcare professional will recommend a needle that is appropriate
for you (needles not included). See enclosed Package Insert and Instructions for Use.
Storage: Refrigerate at 2–8°C (36–46°F). DO NOT FREEZE. PROTECT FROM LIGHT.Rx only
KEEP REFRIGERATED
Genentech
10181030


-
INGREDIENTS AND APPEARANCE
NUTROPIN AQ NUSPIN 5
somatropin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-075 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 5 mg in 2 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 17.4 mg in 2 mL PHENOL (UNII: 339NCG44TV) 5 mg in 2 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 4 mg in 2 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 10 mmol in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-075-01 1 in 1 CARTON 12/29/1995 1 2 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020522 12/29/1995 NUTROPIN AQ NUSPIN 10
somatropin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-074 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 10 mg in 2 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 17.4 mg in 2 mL PHENOL (UNII: 339NCG44TV) 5 mg in 2 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 4 mg in 2 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 10 mmol in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-074-01 1 in 1 CARTON 12/29/1995 1 2 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020522 12/29/1995 NUTROPIN AQ NUSPIN 20
somatropin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-076 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 20 mg in 2 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 17.4 mg in 2 mL PHENOL (UNII: 339NCG44TV) 5 mg in 2 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 4 mg in 2 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 10 mmol in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-076-01 1 in 1 CARTON 12/29/1995 1 2 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020522 12/29/1995 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 ANALYSIS(50242-074, 50242-075, 50242-076) , API MANUFACTURE(50242-074, 50242-075, 50242-076) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 833220176 PACK(50242-074, 50242-075, 50242-076)