| NDC | 61703-422-78, 61703-422-83, 61703-426-01, 61703-426-02, 61703-430-01, 61703-430-02, 61703-434-01, 61703-434-82 |
| Set ID | e3912909-50c7-4ad2-49b5-ae3a3dfec758 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Hospira, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | NDA021625 |
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use M.V.I. ADULT™ safely and effectively. See full prescribing information for M.V.I. ADULT.
M.V.I. ADULT (multiple vitamins injection), for intravenous use
Initial U.S. Approval: 2004INDICATIONS AND USAGE
M.V.I. Adult is a combination of vitamins indicated for prevention of vitamin deficiency in adults and pediatric patients aged 11 years and above receiving parenteral nutrition (1)
DOSAGE AND ADMINISTRATION
- •
- M.V.I. Adult is a combination product that contains the following vitamins: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K, folic acid, biotin, and vitamin B12 (2.1)
- •
- Recommended daily dosage is 10 mL (2.2)
- •
- Administer by intravenous infusion after dilution (2.1)
- •
- Supplied as a single-dose and a pharmacy bulk package:
- •
- M.V.I. Adult Single Dose: consists of two vials labeled Vial 1 and Vial 2. Transfer contents of Vial 1 to Vial 2. The mixed solution (10 mL) will provide a single 10 mL dose. Use within 4 hours of puncture (2.1, 2.3)
- •
- M.V.I.Adult Pharmacy Bulk Package: consists of two vials labeled Vial 1 and Vial 2. Transfer the contents of Vial 1 to Vial 2. The mixed solution (100 mL) will provide ten 10 mL single doses to patients in a pharmacy admixture program. Use within 4 hours of puncture (2.1, 2.3)
- •
- Prior to intravenous administration, dilute the once daily dose of 10 mL by adding to at least 500 to 1,000 mL intravenous parenteral nutrition solution containing dextrose or saline (2.3)
- •
- After dilution in an intravenous infusion, refrigerate resulting solution unless used immediately. Use solution within 24 hours after dilution (2.3)
- •
- Monitor blood vitamin concentrations (2.4)
- •
- See Full Prescribing Information for drug incompatibilities (2.5)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Decreased Anticoagulant Effect of Warfarin: Periodically monitor prothrombin time/INR. (5.1)
- •
- Risk of Aluminum Toxicity: For at risk patients (renal failure or those with prolonged therapy), periodically monitor aluminum levels with prolonged administration (5.2)
- •
- Low Vitamin A levels: Monitor vitamin A levels (5.3)
- •
- Allergic Reactions: to thiamine may occur (5.4)
- •
- Hypervitaminosis A: Patients with renal failure or liver disease may be at higher risk (5.5)
- •
- Interferes with Megaloblastic Anemia Diagnosis: Avoid use during testing for this disorder (5.6)
- •
- Risk of Vitamin Deficiencies or Excess: Monitor blood vitamin concentrations (5.7)
- •
- False Negative Urine Glucose Tests: May occur due to vitamin C (5.8)
ADVERSE REACTIONS
Adverse reactions have included anaphylaxis, rash, erythema, pruritus, headache, dizziness, agitation, anxiety, diplopia (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Effect of M.V.I. Adult on other drugs:
- •
- Phenytoin: Folic acid may decrease phenytoin blood levels and increase the risk of seizure activity (7.1)
- •
- Methotrexate: Folic acid may decrease response to methotrexate (7.1)
- •
- Levodopa: Pyridoxine may decrease blood levels of levodopa and levodopa efficacy may decrease (7.1)
- •
- Antibiotics: Thiamine, riboflavin, pyridoxine, niacinamide, and ascorbic acid decrease activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin (7.1)
- •
- Bleomycin: Ascorbic acid and riboflavin may reduce the activity of bleomycin (7.1)
Effects of other drugs on M.V.I. Adult:
- •
- Hydralazine, Isoniazid: Concomitant administration of hydralazine or isoniazid may increase pyridoxine requirements (7.2)
- •
- Chloramphenicol: In patients with pernicious anemia, hematologic response to vitamin B12 may be inhibited by concomitant administration of chloramphenicol (7.2)
- •
- Phenytoin: May decrease folic acid concentrations (7.2)
USE IN SPECIFIC POPULATIONS
- •
- Pregnant and Nursing Mothers: Pregnant and nursing women should follow the U.S. Recommended Daily Allowances for their condition, because their vitamin requirements may exceed those of nonpregnant and nonlactating women (8.1, 8.3)
- •
- Pediatric Use: Safety and effectiveness in pediatric patients below the age of 11 years have not been established (8.4)
- •
- Renal Impairment: Monitor renal function, calcium, phosphorus and vitamin A levels (8.6)
- •
- Hepatic Impairment: Monitor vitamin A levels (8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Dosage Information
2.3 Preparation and Administration Instructions
2.4 Monitoring Vitamin Blood Levels
2.5 Drug Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Anticoagulant Effect of Warfarin
5.2 Aluminum Toxicity
5.3 Risk of Low Vitamin A Levels
5.4 Allergic Reactions to Thiamine
5.5 Hypervitaminosis A
5.6 Interference with Diagnosis of Megaloblastic Anemia
5.7 Potential to Develop Vitamin Deficiencies or Excesses
5.8 Interference with Urine Glucose Testing
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effect of M.V.I. Adult on Other Drugs
7.2 Effect of Other Drugs on M.V.I. Adult
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
M.V.I. Adult is a combination product that contains the following vitamins: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K, folic acid, biotin, and vitamin B12.
M.V.I. Adult is supplied as a single dose or as a pharmacy bulk package for intravenous use intended for administration by intravenous infusion after dilution:
- •
- M.V.I. Adult Single Dose: consists of two vials which must be mixed prior to use. The mixed solution will provide a single 10 mL dose which must be diluted prior to intravenous administration [see Dosage and Administration (2.3)].
- •
- M.V.I. Adult Pharmacy Bulk Package: consists of two pharmacy bulk vials which must be mixed prior to use. The mixed solution will provide ten 10 mL single doses which must be diluted prior to intravenous administration. Pharmacy bulk package of M.V.I. Adult is intended for dispensing of single doses to multiple patients in a pharmacy admixture program and is restricted to the preparation of admixtures for infusion [see Dosage and Administration (2.3)].
Do not administer M.V.I. Adult as a direct, undiluted intravenous injection as it may cause dizziness, faintness, and tissue irritation.
2.2 Dosage Information
The recommended daily dosage volume is 10 mL. One daily dose (10 mL) is diluted by adding directly to a specified volume of an intravenous fluid [see Dosage and Administration (2.3)].
Patients with multiple vitamin deficiencies or with increased vitamin requirements may need multiple daily dosages as indicated or additional doses of individual vitamins.
2.3 Preparation and Administration Instructions
M.V.I. Adult supplied as a single dose:
- •
- M.V.I Adult is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
- •
- Transfer the contents of Vial 1 (5 mL of solution) into the contents of Vial 2 (5 mL of solution). The mixed solution (10 mL) will provide a single 10 mL dose.
- •
- Once the closure system has been penetrated, complete withdrawal of vial contents within 4 hours. Mixed solution may be stored for up to 4 hours refrigerated.
- •
- Visually inspect for particulate matter and discoloration prior to intravenous administration.
- •
- Utilizing a suitable sterile automated compounding device or dispensing pin for accuracy, aseptically transfer the 10 mL dose into a plastic or glass bottle containing at least 500 to 1,000 mL intravenous parenteral nutrition solution containing dextrose or saline.
- •
- After M.V.I. Adult is diluted in an intravenous infusion, refrigerate the resulting solution unless it is to be used immediately, and use the solution within 24 hours after dilution.
- •
- Minimize exposure to light because some of the vitamins in M.V.I. Adult, particularly A, D and riboflavin, are light sensitive.
M.V.I. Adult supplied as a pharmacy bulk package:
- •
- M.V.I. Adult is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
- •
- Transfer the contents of Vial 1 (50 mL) into Vial 2 (50 mL). The mixed solution (100 mL) will provide ten 10 mL single doses to patients in a pharmacy admixture program.
- •
- Each bulk vial closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set that allows measured dispensing of the contents.
- •
- Once the closure system has been penetrated, complete dispensing from the pharmacy bulk vial within 4 hours. Mixed solution may be stored for up to 4 hours refrigerated.
- •
- Discard unused portion.
- •
- Visually inspect for particulate matter and discoloration prior to administration.
- •
- Utilizing a suitable sterile automated compounding device or dispensing pin for accuracy, aseptically transfer each 10 mL dose into a plastic or glass bottle containing at least 500 to 1,000 mL intravenous parenteral nutrition solution containing dextrose or saline.
- •
- After M.V.I. Adult is diluted in an intravenous infusion, refrigerate the resulting solution unless it is to be used immediately, and use the solution within 24 hours after dilution.
- •
- Minimize exposure to light because some of the vitamins in M.V.I. Adult, particularly A, D and riboflavin, are light sensitive.
2.4 Monitoring Vitamin Blood Levels
Blood vitamin concentrations should be monitored to ensure maintenance of adequate levels, particularly in patients receiving parenteral multivitamins as the only source of vitamins for long periods of time.
2.5 Drug Incompatibilities
- •
- M.V.I. Adult is not physically compatible with moderately alkaline solutions such as a sodium bicarbonate solution and other alkaline drugs such as acetazolamide sodium, aminophylline, ampicillin sodium, and chlorothiazide sodium.
- •
- Folic acid is unstable in the presence of calcium salts such as calcium gluconate.
- •
- Vitamin A and thiamine in M.V.I.Adult may react with bisulfite solutions such as sodium bisulfite or vitamin K bisulfite. Do not add M.V.I. Adult directly to intravenous fat emulsions.
- •
- Consult appropriate references for listings of physical and chemical compatibility of solutions and drugs with M.V.I. Adult. In such circumstances, admixture or Y-site administration with M.V.I. Adult should be avoided.
-
3 DOSAGE FORMS AND STRENGTHS
M.V.I. Adult is an injection available as a:
- •
- Single dose: consisting of two vials labeled Vial 1 and Vial 2. Vial 1 is an amber vial containing a clear, amber to orange colored solution. Vial 2 is an amber vial containing a clear to light straw colored solution. Both vials must be mixed prior to use. The mixed solution (10 mL) will provide a single 10 mL dose [see Dosage and Administration (2.3) and Description (11)].
- •
- Pharmacy bulk package: consisting of two vials labeled Vial 1 and Vial 2. Vial 1 is an amber vial containing a clear, amber to orange colored solution. Vial 2 is an amber vial containing a clear to light straw colored solution. Both vials must be mixed prior to use. The mixed solution (100 mL) will provide ten 10 mL single doses [see Dosage and Administration (2.3))].
See Description section for vitamin strengths [see Description (11)].
-
4 CONTRAINDICATIONS
M.V.I. Adult is contraindicated in patients who have:
- •
- A history of known hypersensitivity to any of the vitamins or excipients in M.V.I. Adult [see Warnings and Precautions (5.4), Adverse Reactions (6)]
- •
- An existing hypervitaminosis
-
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Anticoagulant Effect of Warfarin
M.V.I. Adult contains Vitamin K which may decrease the anticoagulant effect of warfarin. In patients who are on warfarin anticoagulant therapy receiving M.V.I. Adult, prothrombin time/INR should be periodically monitored to determine if the dose of warfarin needs to be adjusted.
5.2 Aluminum Toxicity
M.V.I. Adult contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration in patients with renal impairment. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 micrograms per kg per day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration. To prevent aluminum toxicity monitor periodically aluminum levels with prolonged parenteral administration of M.V.I. Adult.
5.3 Risk of Low Vitamin A Levels
Vitamin A may adhere to plastic, resulting in lower vitamin A concentrations after administration of M.V.I. Adult. Therefore, blood vitamin concentrations should be periodically monitored and the administration of additional therapeutic doses of Vitamin A may be required.
5.4 Allergic Reactions to Thiamine
Allergic reactions such as urticaria, periorbital and digital edema, have been reported following intravenous administration of thiamine, which is found in M.V.I. Adult. There have been rare reports of anaphylaxis following intravenous doses of thiamine. No fatal anaphylaxis reactions associated with M.V.I. Adult have been reported.
5.5 Hypervitaminosis A
Hypervitaminosis A, manifested by nausea, vomiting, headache, dizziness, blurred vision, has been reported in patients with renal failure receiving 1.5 mg/day retinol and in patients with liver disease. Therefore, supplementation of renal failure patients and patients with liver diseases with vitamin A, an ingredient found in M.V.I. Adult, should be undertaken with caution [see Use in Specific Populations (8.6, 8.7)]. Blood levels of Vitamin A should be monitored periodically.
5.6 Interference with Diagnosis of Megaloblastic Anemia
M.V.I. Adult contains folic acid and cyanocobalamin which can mask serum deficits of folic acid and cyanocobalamin in patients with megaloblastic anemia. Avoid the use of M.V.I. Adult in patients with suspected or diagnosed megaloblastic anemia prior to blood sampling for the detection of the folic acid and cyanocobalamin deficiencies.
5.7 Potential to Develop Vitamin Deficiencies or Excesses
In patients receiving parenteral multivitamins, such as with M.V.I. Adult, blood vitamin concentrations should be periodically monitored to determine if vitamin deficiencies or excesses are developing. M.V.I. Adult may not correct long-standing specific vitamin deficiencies. The administration of additional doses of specific vitamins may be required [see Dosage and Administration (2.2)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other section of the labeling.
- •
- Allergic Reactions to Thiamine [see Warnings and Precautions (5.4)].
- •
- Hypervitaminosis A [see Warnings and Precautions (5.5)].
The following adverse reactions have been identified during post approval use of M.V.I. Adult. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: rash, erythema, pruritus
CNS: headache, dizziness, agitation, anxiety
Ophthalmic: diplopia -
7 DRUG INTERACTIONS
7.1 Effect of M.V.I. Adult on Other Drugs
Phenytoin: Folic acid may increase phenytoin metabolism and lower the serum concentration of phenytoin resulting in increased seizure activity.
Methotrexate: Folic acid may decrease a patient's response to methotrexate therapy.
Levodopa: Pyridoxine may increase the metabolism of levodopa (decrease blood level of levodopa) and decrease its efficacy.
Antibiotics: Thiamine, riboflavin, pyridoxine, niacinamide, and ascorbic acid decrease antibiotic activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin.
Bleomycin: Ascorbic acid and riboflavin inactivate bleomycin in vitro, thus the activity of bleomycin may be reduced.
7.2 Effect of Other Drugs on M.V.I. Adult
Hydralazine or Isoniazid:
Concomitant administration of hydralazine or isoniazid may increase pyridoxine requirements.
Chloramphenicol:
In patients with pernicious anemia, the hematologic response to vitamin B12 therapy may be inhibited by concomitant administration of chloramphenicol.
Phenytoin:
Phenytoin may decrease serum folic acid concentrations.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
M.V.I. Adult has not been studied in pregnant women. Pregnant women should follow the U.S. recommended daily allowances for pregnancy because their vitamin requirements may exceed those of nonpregnant women. Animal reproduction studies have not been conducted with M.V.I. Adult (multiple vitamins injection) administered by intravenous infusion.
8.3 Nursing Mothers
M.V.I. Adult has not been studied in lactating women. Lactating women should follow the U.S. Recommended Daily Allowances for their condition, because their vitamin requirements may exceed those of nonlactating women. Caution should be exercised when M.V.I. Adult is administered to a nursing woman.
8.4 Pediatric Use
M.V.I. Adult is indicated for the prevention of vitamin deficiency in pediatric patients aged 11 years and older receiving parenteral nutrition. M.V.I. Adult is not indicated for use in pediatric patients below the age of 11 years.
8.5 Geriatric Use
Reported clinical experience has not identified differences in responses between the elderly and younger patients.
8.6 Renal Impairment
M.V.I. Adult has not been studied in patients with renal impairment. Monitor renal function, calcium, phosphorus and vitamin A levels in patients with renal impairment [see Warnings and Precautions (5.2, 5.5)].
8.7 Hepatic Impairment
M.V.I. Adult has not been studied in patients with hepatic impairment. Monitor vitamin A level in patients with liver disease or high alcohol consumption [see Warnings and Precautions (5.5)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
M.V.I. Adult (multiple vitamins injection) is a sterile product consisting of two vials provided as a single dose or as a pharmacy bulk package for intravenous use intended for administration by intravenous infusion after dilution.
Table 1 provides the strengths of the vitamins provided in vial 1 and vial 2:
Table 1: M.V.I. ADULT FORMULATION (INTENDED FOR AGES 11 AND OLDER) Vial 1*
Fat Soluble Vitamins**
Ingredient
Amount per Unit Dose
Vitamin A (retinol)
1 mga
Vitamin D (ergocalciferol)
5 mcgb
Vitamin E (dl-alpha-tocopheryl acetate)
10 mgc
Vitamin K (phytonadione)
150 mcg
Water Soluble Vitamins
Vitamin C (ascorbic acid)
200 mg
Niacinamide
40 mg
Vitamin B2 (as riboflavin 5-phosphate sodium)
3.6 mg
Vitamin B1 (thiamine)
6 mg
Vitamin B6 (pyridoxine HCl)
6 mg
Dexpanthenol (d-pantothenyl alcohol)
15 mg
* With 30% propylene glycol and 2% gentisic acid ethanolamide as stabilizers and preservatives; sodium hydroxide for pH adjustment; 1.6% polysorbate 80; 0.028% polysorbate 20; 0.002% butylated hydroxytoluene; 0.0005% butylated hydroxyanisole.
** Fat soluble vitamins A, D, E and K are water solubilized with polysorbate 80.
(a) 1 mg vitamin A equals 3,300 USP units.
(b) 5 mcg ergocalciferol equals 200 USP units.
(c) 10 mg vitamin E equals 10 USP units.Vial 2*
Biotin
60 mcg
Folic acid
600 mcg
B12 (cyanocobalamin)
5 mcg
* With 30% propylene glycol; and citric acid, sodium citrate, and sodium hydroxide for pH adjustment.
Multiple vitamin preparation for intravenous infusion:
M.V.I. Adult (multiple vitamins injection) makes available a combination of fat-soluble and water-soluble vitamins in an aqueous solution, formulated for incorporation into intravenous infusions. The liposoluble vitamins A, D, E, and K have been solubilized in an aqueous medium with polysorbate 80, permitting intravenous administration of these vitamins.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
M.V.I. ADULT is an injection supplied in the following package configurations:
M.V.I. ADULT Single-Dose
Unit of Sale
Each Box
Each
Vial 1
Each
Vial 2
NDC 61703-434-82
Bundle of 10 Boxes of
2 vials (Vial 1 and
Vial 2)NDC 61703-434-01
Box of 2 vials
(Vial 1 and Vial 2)NDC 61703-426-02
10 Vitamin Blend,
5 mLNDC 61703-430-02
3 Vitamin Blend,
5 mLVial 1 is an amber vial containing a clear, amber to orange colored solution. Vial 2 is an amber vial containing a clear to light straw colored solution. Mix contents of Vial 1 and Vial 2 to provide a single 10 mL dose [see Dosage and Administration (2.3)].
M.V.I. ADULT Pharmacy Bulk Package
Unit of Sale
Intermediate
Multi-Pack
Each
Vial 1
Each
Vial 2
NDC 61703-422-83
Case of 2 Boxes of
10 vials (5 Vial 1 and
5 Vial 2)NDC 61703-422-78
Box of 10 vials
(5 Vial 1 and 5 Vial 2)NDC 61703-426-01
10 Vitamin Blend,
50 mLNDC 61703-430-01
3 Vitamin Blend,
50 mLVial 1 is an amber vial containing a clear, amber to orange colored solution. Vial 2 is an amber vial containing a clear to light straw colored solution. Mix contents of Vial 1 and Vial 2 to provide ten 10 mL single doses [see Dosage and Administration (2.3)].
See Description section for vitamin strengths [see Description (11)].
Minimize the exposure of M.V.I. Adult to light, because vitamins A, D and riboflavin are light sensitive.
Store at 2-8°C (36-46°F).
-
17 PATIENT COUNSELING INFORMATION
Instruct patients (if age appropriate) and caregivers:
- •
- To watch for and immediately report signs of allergic reactions (i.e. urticaria, periorbital and digital edema).
- •
- To watch for and immediately report signs of hypervitaminosis A, manifested by nausea, vomiting, headache, dizziness, blurred vision, if patients have renal impairment.
- •
- To report other adverse reactions such as rash, erythema, pruritus, headache, dizziness, agitation, anxiety, and diplopia.
- •
- That patients on warfarin anticoagulant therapy will be monitored periodically for blood prothrombin/ INR levels to determine if the dose of warfarin needs to be adjusted.
- •
- About the significance of periodic monitoring of blood vitamin concentrations to determine if vitamin deficiencies or excesses are developing .
- •
- About the need to monitor renal function, calcium, phosphorus, aluminum, and vitamin A levels in patients with renal impairment.
Manufactured by

Hospira, Inc., Lake Forest, IL 60045 USALAB-0989-2.0
-
PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 61703-422-78
Sterile
Contents: 10 vials, 50 mL Fill each
(5 Vial 1 and 5 Vial 2)
Mix contents of Vial 1 with Vial 2 to provide 10 single doses.NDC 61703-422-78
Rx only
M.V.I. ADULT™
(multiple vitamins injection)PHARMACY BULK PACKAGE. NOT FOR DIRECT INFUSION.
See insert for important information on pharmacy bulk package.
Store under refrigeration, 2 - 8°C (36 - 46°F). Store Upright.
Hospira

- PRINCIPAL DISPLAY PANEL - 50 mL Vial Label - NDC 61703-426-01
-
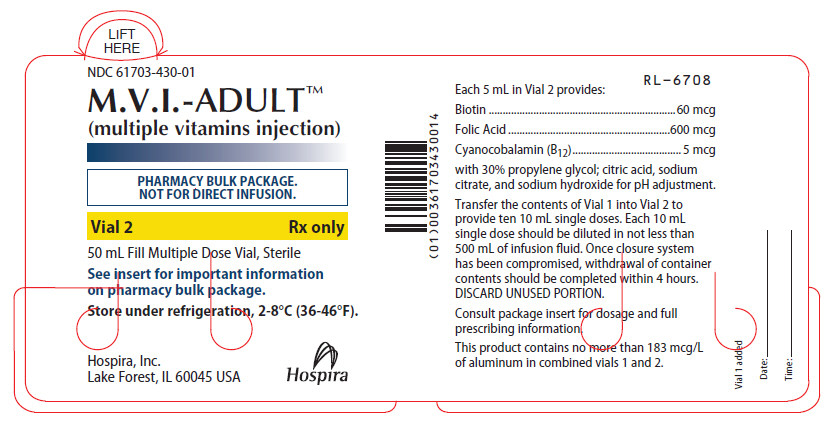
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label - NDC 61703-430-01
LIFT
HERENDC 61703-430-01
M.V.I.-ADULT™
(multiple vitamins injection)PHARMACY BULK PACKAGE.
NOT FOR DIRECT INFUSION.Vial 2
Rx only50 mL Fill Multiple Dose Vial, Sterile
See insert for important information
on pharmacy bulk package.Store under refrigeration, 2-8°C (36-46°F).
Hospira, Inc.
Lake Forest, IL 60045 USAHospira

- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 61703-434-01
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 61703-426-02
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 61703-430-02
-
INGREDIENTS AND APPEARANCE
M.V.I. ADULT
retinol, ergocalciferol, .alpha.-tocopherol acetate, dl-, phytonadione, ascorbic acid, niacinamide, riboflavin 5-phosphate sodium, thiamine hydrochloride, pyridoxine hydrochloride, dexpanthenol, biotin, folic acid, and cyanocobalamin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61703-422 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-422-83 2 in 1 CASE 01/27/2015 1 NDC:61703-422-78 5 in 1 BOX 1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 50 mL Part 2 1 VIAL, MULTI-DOSE 50 mL Part 1 of 2 RETINOL, ERGOCALCIFEROL, .ALPHA.-TOCOPHEROL ACETATE, DL-, PHYTONADIONE, ASCORBIC ACID, NIACINAMIDE, RIBOFLAVIN 5-PHOSPHATE SODIUM, THIAMINE HYDROCHLORIDE, PYRIDOXINE HYDROCHLORIDE, AND DEXPANTHENOL
retinol, ergocalciferol, .alpha.-tocopherol acetate, dl-, phytonadione, ascorbic acid, niacinamide, riboflavin 5-phosphate sodium, thiamine hydrochloride, pyridoxine hydrochloride, and dexpanthenol injection, solution, concentrateProduct Information Item Code (Source) NDC:61703-426 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RETINOL (UNII: G2SH0XKK91) (RETINOL - UNII:G2SH0XKK91) RETINOL 1 mg in 5 mL ERGOCALCIFEROL (UNII: VS041H42XC) (ERGOCALCIFEROL - UNII:VS041H42XC) ERGOCALCIFEROL 5 ug in 5 mL .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 10 mg in 5 mL PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 150 ug in 5 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 200 mg in 5 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 40 mg in 5 mL RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (FLAVIN MONONUCLEOTIDE - UNII:7N464URE7E) FLAVIN MONONUCLEOTIDE 3.6 mg in 5 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 6 mg in 5 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 6 mg in 5 mL DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GENTISIC ACID ETHANOLAMIDE (UNII: H4E039OIGX) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-426-01 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021643 01/27/2015 Part 2 of 2 BIOTIN, FOLIC ACID, AND CYANOCOBALAMIN
biotin, folic acid, and cyanocobalamin injection, solution, concentrateProduct Information Item Code (Source) NDC:61703-430 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 60 ug in 5 mL FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 600 ug in 5 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 5 ug in 5 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-430-01 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021643 01/27/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021643 01/27/2015 M.V.I. ADULT
retinol, ergocalciferol, .alpha.-tocopherol acetate, dl-, phytonadione, ascorbic acid, niacinamide, riboflavin 5-phosphate sodium, thiamine hydrochloride, pyridoxine hydrochloride, dexpanthenol, biotin, folic acid, and cyanocobalamin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61703-434 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-434-82 10 in 1 PACKAGE 04/28/2017 1 NDC:61703-434-01 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 5 mL Part 2 1 VIAL, SINGLE-DOSE 5 mL Part 1 of 2 RETINOL, ERGOCALCIFEROL, .ALPHA.-TOCOPHEROL ACETATE, DL-, PHYTONADIONE, ASCORBIC ACID, NIACINAMIDE, RIBOFLAVIN 5-PHOSPHATE SODIUM, THIAMINE HYDROCHLORIDE, PYRIDOXINE HYDROCHLORIDE, AND DEXPANTHENOL
retinol, ergocalciferol, .alpha.-tocopherol acetate, dl-, phytonadione, ascorbic acid, niacinamide, riboflavin 5-phosphate sodium, thiamine hydrochloride, pyridoxine hydrochloride, and dexpanthenol injection, solution, concentrateProduct Information Item Code (Source) NDC:61703-426 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RETINOL (UNII: G2SH0XKK91) (RETINOL - UNII:G2SH0XKK91) RETINOL 1 mg in 5 mL ERGOCALCIFEROL (UNII: VS041H42XC) (ERGOCALCIFEROL - UNII:VS041H42XC) ERGOCALCIFEROL 5 ug in 5 mL .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 10 mg in 5 mL PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 150 ug in 5 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 200 mg in 5 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 40 mg in 5 mL RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (FLAVIN MONONUCLEOTIDE - UNII:7N464URE7E) FLAVIN MONONUCLEOTIDE 3.6 mg in 5 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 6 mg in 5 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 6 mg in 5 mL DEXPANTHENOL (UNII: 1O6C93RI7Z) (DEXPANTHENOL - UNII:1O6C93RI7Z) DEXPANTHENOL 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GENTISIC ACID ETHANOLAMIDE (UNII: H4E039OIGX) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-426-02 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021625 04/28/2017 Part 2 of 2 BIOTIN, FOLIC ACID, AND CYANOCOBALAMIN
biotin, folic acid, and cyanocobalamin injection, solution, concentrateProduct Information Item Code (Source) NDC:61703-430 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 60 ug in 5 mL FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 600 ug in 5 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 5 ug in 5 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61703-430-02 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021625 04/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021625 04/28/2017 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(61703-422, 61703-434) , LABEL(61703-422, 61703-434) , MANUFACTURE(61703-422, 61703-434) , PACK(61703-422, 61703-434)