| NDC | 44946-2017-8, 44946-2018-8 |
| Set ID | 1122efb5-a9bf-4641-a427-f1a99bfae7a0 |
| Category | DIETARY SUPPLEMENT |
| Packager | Sancilio & Company, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
STATEMENT OF IDENTITY
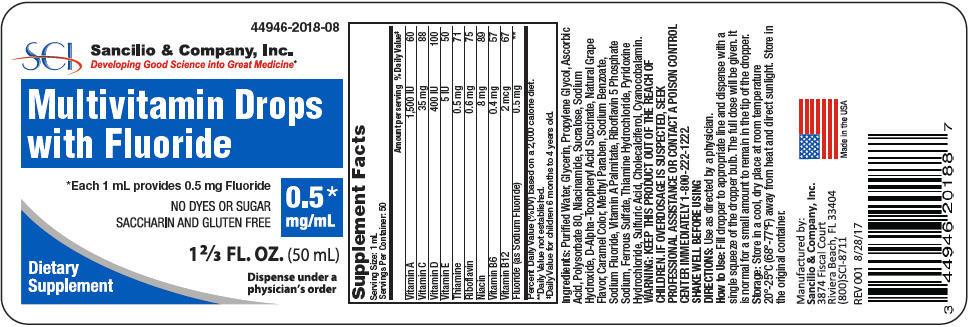
Supplement Facts Serving Size: 1 mL Servings Per Container: 50 Amount per serving % Daily Value* Percent Daily Value (%DV) based on a 2,000 calorie diet. Vitamin A 1,500 IU 60 Vitamin C 35 mg 88 Vitamin D 400 IU 100 Vitamin E 5 IU 50 Thiamine 0.5 mg 71 Riboflavin 0.6 mg 75 Niacin 8 mg 89 Vitamin B6 0.4 mg 57 Vitamin B12 2 mcg 67 Fluoride (as Sodium Fluoride) 0.5 mg † Ingredients: Purified Water, Glycerin, Propylene Glycol, Ascorbic Acid, Polysorbate 80, Niacinamide, Sucralose, Sodium Hydroxide, D-Alpha-Tocopheryl Acid Succinate, Natural Grape Flavor, Caramel Color, Methyl Paraben, Sodium Benzoate, Sodium Fluoride, Vitamin A Palmitate, Riboflavin 5 Phosphate Sodium, Ferrous Sulfate, Thiamine Hydrochloride, Pyridoxine Hydrochloride, Sulfuric Acid, Cholecalciferol, Cyanocobalamin.
- WARNING
- DIRECTIONS
- Storage
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 0.25 mg/mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 0.5 mg/mL Bottle Label
-
INGREDIENTS AND APPEARANCE
MULTIVITAMIN DROPS WITH FLUORIDE
vitamin a palmitate, ascorbic acid, cholecalciferol, tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, cyanocobalamin, and sodium fluoride liquidProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:44946-2017 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vitamin A Palmitate (UNII: 1D1K0N0VVC) (Vitamin A - UNII:81G40H8B0T) Vitamin A 1500 [iU] in 1 mL Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 35 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] in 1 mL TOCOPHEROL (UNII: R0ZB2556P8) (TOCOPHEROL - UNII:R0ZB2556P8) TOCOPHEROL 5 [iU] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 0.5 mg in 1 mL RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (FLAVIN MONONUCLEOTIDE - UNII:7N464URE7E) FLAVIN MONONUCLEOTIDE 0.6 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 8 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.4 mg in 1 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 2 ug in 1 mL Sodium Fluoride (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Propylene Glycol (UNII: 6DC9Q167V3) Polysorbate 80 (UNII: 6OZP39ZG8H) Sodium Hydroxide (UNII: 55X04QC32I) Sucralose (UNII: 96K6UQ3ZD4) Methylparaben (UNII: A2I8C7HI9T) Caramel (UNII: T9D99G2B1R) Sodium Benzoate (UNII: OJ245FE5EU) Sulfuric Acid (UNII: O40UQP6WCF) Ferrous Sulfate (UNII: 39R4TAN1VT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:44946-2017-8 50 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 08/24/2017 MULTIVITAMIN DROPS WITH FLUORIDE
vitamin a palmitate, ascorbic acid, cholecalciferol, tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, cyanocobalamin, and sodium fluoride liquidProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:44946-2018 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vitamin A Palmitate (UNII: 1D1K0N0VVC) (Vitamin A - UNII:81G40H8B0T) Vitamin A 1500 [iU] in 1 mL Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 35 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] in 1 mL TOCOPHEROL (UNII: R0ZB2556P8) (TOCOPHEROL - UNII:R0ZB2556P8) TOCOPHEROL 5 [iU] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 0.5 mg in 1 mL RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (FLAVIN MONONUCLEOTIDE - UNII:7N464URE7E) FLAVIN MONONUCLEOTIDE 0.6 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 8 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.4 mg in 1 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 2 ug in 1 mL Sodium Fluoride (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Propylene Glycol (UNII: 6DC9Q167V3) Polysorbate 80 (UNII: 6OZP39ZG8H) Sodium Hydroxide (UNII: 55X04QC32I) Sucralose (UNII: 96K6UQ3ZD4) Methylparaben (UNII: A2I8C7HI9T) Caramel (UNII: T9D99G2B1R) Sodium Benzoate (UNII: OJ245FE5EU) Sulfuric Acid (UNII: O40UQP6WCF) Ferrous Sulfate (UNII: 39R4TAN1VT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:44946-2018-8 50 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 08/24/2017 Labeler - Sancilio & Company, Inc. (176681257)