| NDC | 43742-0837-1 |
| Set ID | ec3385e8-7ebc-4d22-a3af-0301d8b860c9 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Deseret Biologicals, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
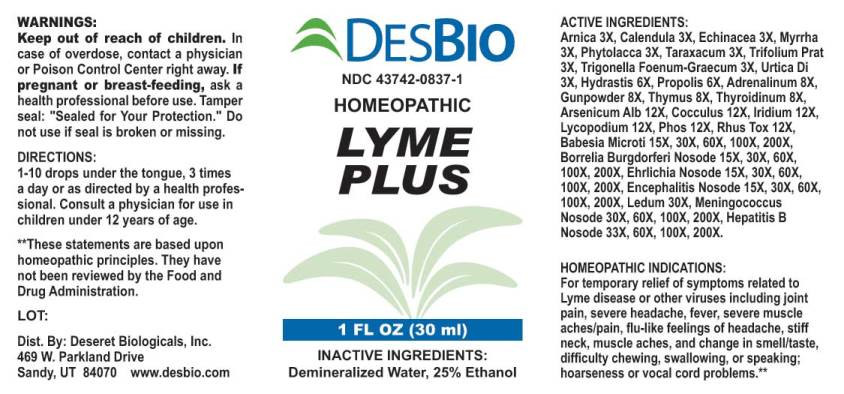
ACTIVE INGREDIENTS:
Arnica Montana 3X, Calendula Officinalis 3XX, Echinacea (Angustifolia) 3X, Myrrha 3X, Phytolacca Decandra 3X, Taraxacum Officinale 3X, Trifolium Pratense 3X, Trigonella Foenum-Graecum 3X, Urtica Dioica 3X, Hydrastis Canadensis 6X, Propolis 6X, Adrenalinum 8X, Gunpowder 8X, Thymus (Suis) 8X, Thyroidinum (Suis) 8X, Arsenicum Album 12X, Cocculus Indicus 12X, Iridium Metallicum 12X, Lycopodium Clavatum 12X, Phosphorus 12X, Rhus Tox 12X, Babesia Microti 15X, 30X, 60X,100X, 200X, Borrelia Burgdorferi Nosode 15X, 30X, 60X,100X, 200X, Ehrlichia Nosode (Canine) 15X, 30X, 60X,100X, 200X, Encephalitis Nosode 15X, 30X, 60X,100X, 200X, Ledum Palustre 30X, Meningococcus Nosode 30X, 60X,100X, 200X, Hepatitis B Nosode 33X, 60X, 100X, 200X.
-
HOMEOPATHIC INDICATIONS:
For temporary relief of symptoms related to Lyme disease or other viruses including joint pain, severe headache, fever, severe muscle aches/pain, flu-like feelings of headache, stiff neck, muscle aches, and change in smell/taste, difficulty chewing, swallowing, or speaking; hoarseness or vocal cord problems.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
-
HOMEOPATHIC INDICATIONS:
For temporary relief of symptoms related to Lyme Disease or other viruses including joint pain, severe headache, fever, severe muscle aches/pain, flu-like feelings of headache, stiff neck, muscle aches, and change in smell/taste, difficulty chewing, swallowing, or speaking; hoarseness or vocal cord problems.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
LYME PLUS
arnica montana, calendula officinalis, echinacea (angustifolia), myrrha, phytolacca decandra, taraxacum officinale, trifolium pratense, trigonella foenum-graecum, urtica dioica, hydrastis canadensis, propolis, adrenalinum, gunpowder, thymus (suis), thyroidinum (suis), arsenicum album, cocculus indicus, iridium metallicum, lycopodium clavatum, phosphorus, rhus tox, babesia microti, borrelia burgdorferi nosode, ehrlichia nosode (canine), encephalitis nosode, ledum palustre, meningococcus nosode liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-0837 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 1 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 1 mL MYRRH (UNII: JC71GJ1F3L) (MYRRH - UNII:JC71GJ1F3L) MYRRH 3 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 3 [hp_X] in 1 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 3 [hp_X] in 1 mL TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 3 [hp_X] in 1 mL FENUGREEK SEED (UNII: 654825W09Z) (FENUGREEK SEED - UNII:654825W09Z) FENUGREEK SEED 3 [hp_X] in 1 mL URTICA DIOICA (UNII: 710FLW4U46) (URTICA DIOICA - UNII:710FLW4U46) URTICA DIOICA 3 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL PROPOLIS WAX (UNII: 6Y8XYV2NOF) (PROPOLIS WAX - UNII:6Y8XYV2NOF) PROPOLIS WAX 6 [hp_X] in 1 mL EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 8 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 8 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 8 [hp_X] in 1 mL POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 8 [hp_X] in 1 mL SUS SCROFA THYMUS (UNII: 7B69B0BD62) (SUS SCROFA THYMUS - UNII:7B69B0BD62) SUS SCROFA THYMUS 8 [hp_X] in 1 mL SUS SCROFA THYROID (UNII: 6RV024OAUQ) (SUS SCROFA THYROID - UNII:6RV024OAUQ) SUS SCROFA THYROID 8 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 12 [hp_X] in 1 mL IRIDIUM (UNII: 44448S9773) (IRIDIUM - UNII:44448S9773) IRIDIUM 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 1 mL BABESIA MICROTI (UNII: 1948X6KEG3) (BABESIA MICROTI - UNII:1948X6KEG3) BABESIA MICROTI 15 [hp_X] in 1 mL BORRELIA BURGDORFERI (UNII: 0J8NV9V5Q8) (BORRELIA BURGDORFERI - UNII:0J8NV9V5Q8) BORRELIA BURGDORFERI 15 [hp_X] in 1 mL EHRLICHIA CANIS (UNII: 970Y8T1JZY) (EHRLICHIA CANIS - UNII:970Y8T1JZY) EHRLICHIA CANIS 15 [hp_X] in 1 mL JAPANESE ENCEPHALITIS VIRUS (UNII: P07E7XWU9D) (JAPANESE ENCEPHALITIS VIRUS - UNII:P07E7XWU9D) JAPANESE ENCEPHALITIS VIRUS 15 [hp_X] in 1 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 30 [hp_X] in 1 mL NEISSERIA MENINGITIDIS (UNII: V3TP2MD7F3) (NEISSERIA MENINGITIDIS - UNII:V3TP2MD7F3) NEISSERIA MENINGITIDIS 30 [hp_X] in 1 mL HEPATITIS B VIRUS (UNII: 77H9EM77P7) (HEPATITIS B VIRUS - UNII:77H9EM77P7) HEPATITIS B VIRUS 33 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-0837-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 08/19/2016 01/29/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/19/2016 01/29/2023 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-0837) , api manufacture(43742-0837) , label(43742-0837) , pack(43742-0837)