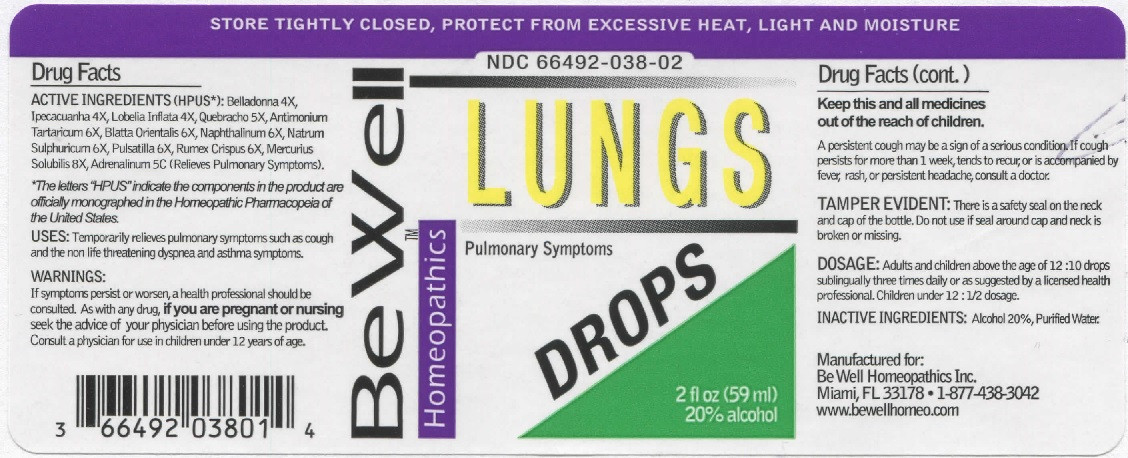
| NDC | 66492-038-02 |
| Set ID | 684459e3-d6ae-4f7f-b823-539cbb9445c1 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS (HPUS*): Belladonna 4X, Ipecacuanha 4X, Lobelia Inflata 4X, Quebracho 5X, Antimonium Tartaricum 6X, Blatta Orientalis 6X, Naphthalinum 6X, Natrum Sulphuricum 6X, Pulsatilla 6X, Rumex Crispus 6X, Mercurius Solubilis 8X, Adrenalinum 5C (Relieves Pulmonary Symptoms).
*The letters "HPUS" indicate that the components in the product are officially monographed in the Homeopathic Pharmacopeia of the United States.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUNG DROPS
belladonna, ipecacuanha, lobelia inflata, quebracho, antimonium tartaricum, blatta orientalis, naphthalinum, natrum sulphuricum, pulsatilla, rumex crispus, mercurius solubilis, adrenalinum. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66492-038 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 4 [hp_X] in 59 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 4 [hp_X] in 59 mL LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 4 [hp_X] in 59 mL ASPIDOSPERMA QUEBRACHO-BLANCO BARK (UNII: 52B1340190) (ASPIDOSPERMA QUEBRACHO-BLANCO BARK - UNII:52B1340190) ASPIDOSPERMA QUEBRACHO-BLANCO BARK 5 [hp_X] in 59 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_X] in 59 mL BLATTA ORIENTALIS (UNII: 535787266D) (BLATTA ORIENTALIS - UNII:535787266D) BLATTA ORIENTALIS 6 [hp_X] in 59 mL NAPHTHALENE (UNII: 2166IN72UN) (NAPHTHALENE - UNII:2166IN72UN) NAPHTHALENE 6 [hp_X] in 59 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 6 [hp_X] in 59 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 59 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_X] in 59 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 8 [hp_X] in 59 mL EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 5 [hp_C] in 59 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66492-038-02 59 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/25/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/25/2014 Labeler - Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics (052584997)