| NDC | 43419-751-31, 43419-752-31, 43419-752-36, 43419-753-31, 43419-753-36, 43419-754-31, 43419-755-31, 43419-756-31 |
| Set ID | cf6c1c52-801f-4eb9-b0f9-2b9a56da4f84 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Amorepacific Corporation |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- Drug Facts
- ACTIVE INGREDIENTS
- PURPOSE
-
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- WARNINGS
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
- To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m.- 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses. Children under 6 months of age: Ask a doctor.
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
WATER / AQUA / EAU, CYCLOPENTASILOXANE, TITANIUM DIOXIDE (CI 77891), PEG-10 DIMETHICONE, CYCLOHEXASILOXANE, PHENYL TRIMETHICONE, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, NIACINAMIDE, ACRYLATES /ETHYLHEXYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, METHYL METHACRYLATE CROSSPOLYMER, BUTYLENE GLYCOL, SODIUM CHLORIDE, ALUMINUM HYDROXIDE, IRON OXIDES (CI 77492), HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, STEARIC ACID, TRIETHOXYCAPRYLYLSILANE, PHENOXYETHANOL, FRAGRANCE / PARFUM, DISTEARDIMONIUM HECTORITE, CAPRYLYL GLYCOL, YEAST EXTRACT / FAEX / EXTRAIT DE LEVURE, ACRYLATES/STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, IRON OXIDES (CI 77491), DIMETHICONE, TRIMETHYLSILOXYSILICATE, POLYSORBATE 80, DISODIUM EDTA, IRON OXIDES (CI 77499), HYDROGENATED LECITHIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, MICA (CI 77019), SILICA, 1,2-HEXANEDIOL, POLYPROPYLSILSESQUIOXANE, CHENOPODIUM QUINOA SEED EXTRACT, MAGNESIUM SULFATE, CALCIUM CHLORIDE, CAMELLIA SINENSIS LEAF EXTRACT, MANGANESE SULFATE, ZINC SULFATE, ASCORBYL GLUCOSIDE
- QUESTIONS?
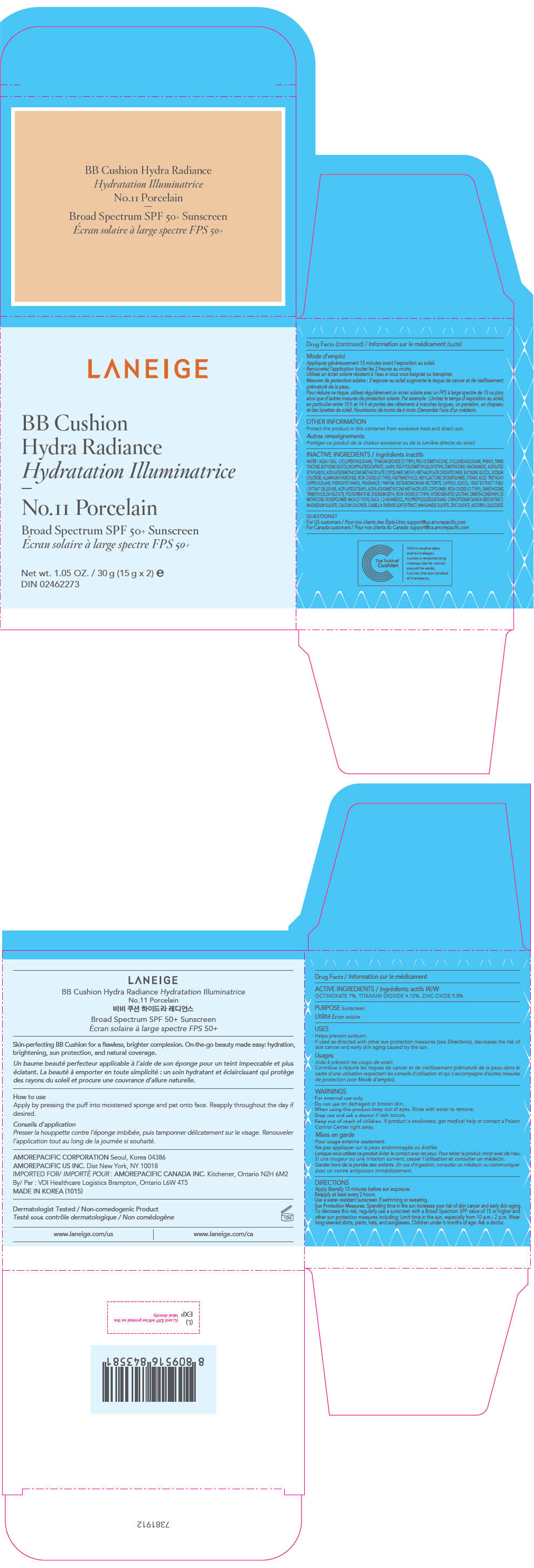
- PRINCIPAL DISPLAY PANEL - 15 g x 2 Container Carton - No.11 Porcelain
- PRINCIPAL DISPLAY PANEL - x 2 (15 g x 2 Container Carton) Carton - No.21 Beige
- PRINCIPAL DISPLAY PANEL - x 2 (15 g x 2 Container Carton) Carton - No.23 Sand
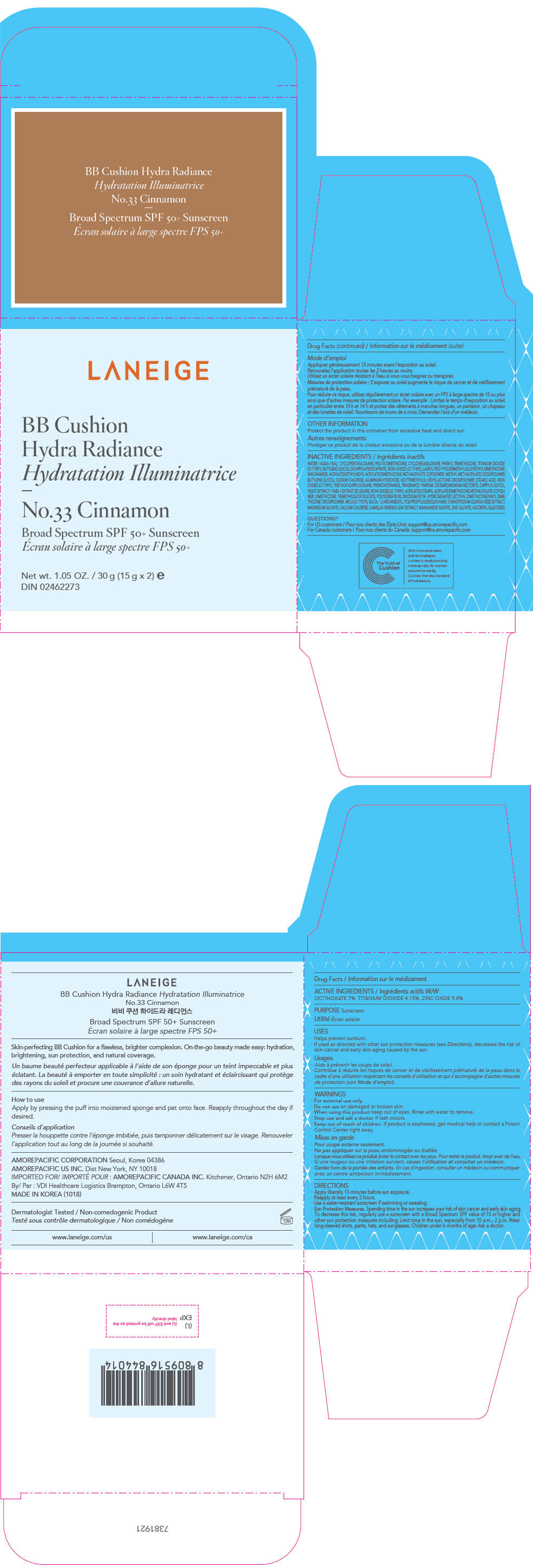
- PRINCIPAL DISPLAY PANEL - 15 g x 2 Container Carton - No.33 Cinnamon
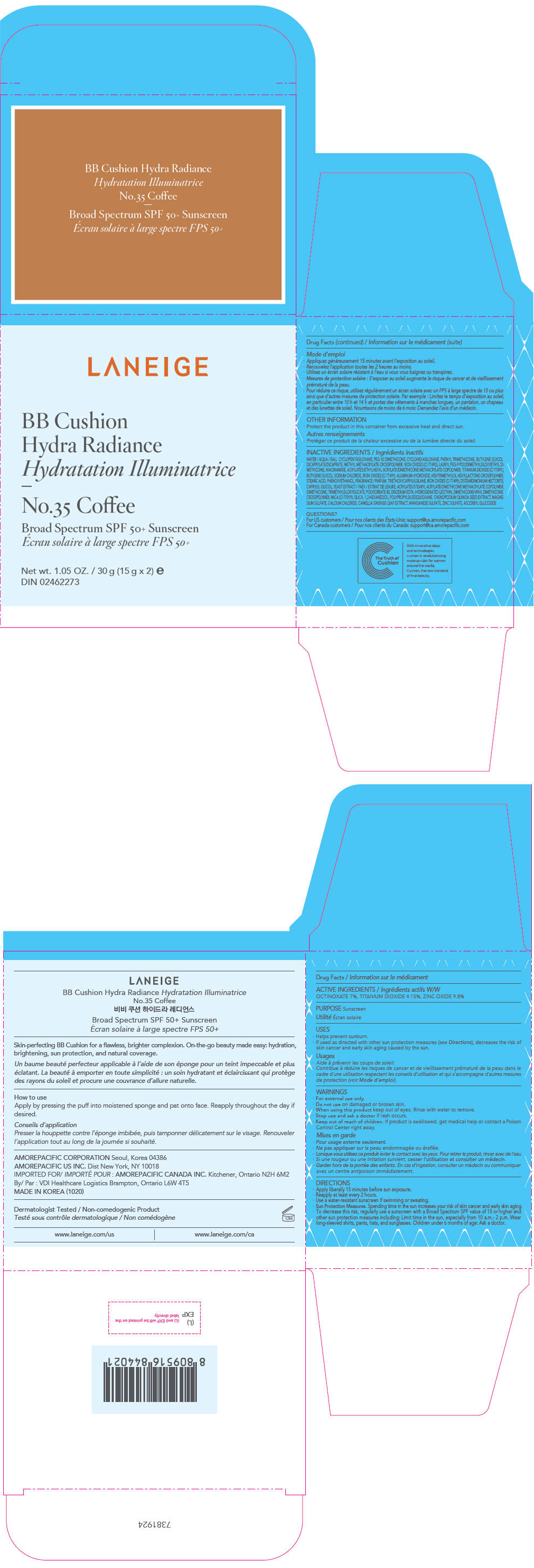
- PRINCIPAL DISPLAY PANEL - 15 g x 2 Container Carton - No.35 Coffee
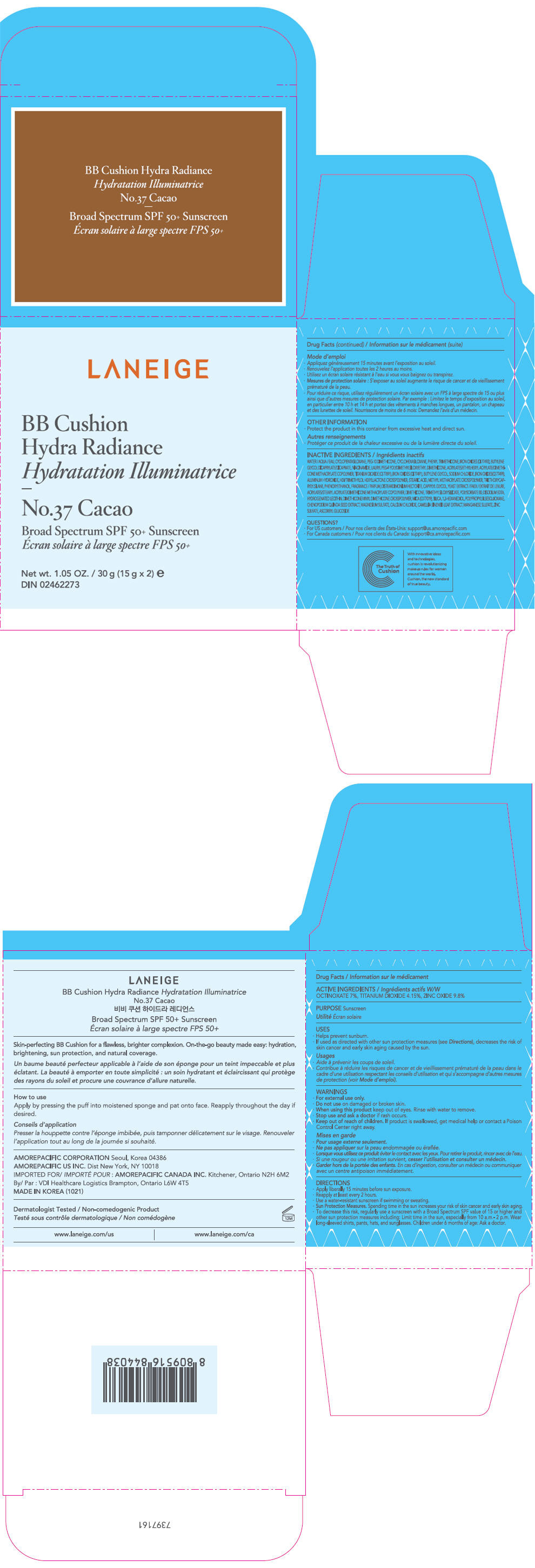
- PRINCIPAL DISPLAY PANEL - 15 g x 2 Container Carton - No.37 Cacao
-
INGREDIENTS AND APPEARANCE
LANEIGE BB CUSHION HYDRA RADIANCE NO.11 PORCELAIN
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-751 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-751-31 2 in 1 CARTON 12/26/2016 11/08/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 11/08/2021 LANEIGE BB CUSHION HYDRA RADIANCE NO.21 BEIGE
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-752 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-752-31 2 in 1 CARTON 12/26/2016 01/10/2020 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:43419-752-36 2 in 1 CARTON 12/26/2016 11/08/2021 2 NDC:43419-752-31 2 in 1 CARTON 2 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 11/08/2021 LANEIGE BB CUSHION HYDRA RADIANCE NO.23 SAND
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-753 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-753-31 2 in 1 CARTON 12/26/2016 01/10/2020 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:43419-753-36 2 in 1 CARTON 12/26/2016 11/08/2021 2 NDC:43419-753-31 2 in 1 CARTON 2 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 11/08/2021 LANEIGE BB CUSHION HYDRA RADIANCE NO.33 CINNAMON
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-754 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-754-31 2 in 1 CARTON 12/26/2016 12/16/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 12/16/2021 LANEIGE BB CUSHION HYDRA RADIANCE NO.35 COFFEE
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-755 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-755-31 2 in 1 CARTON 12/26/2016 04/18/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 04/18/2021 LANEIGE BB CUSHION HYDRA RADIANCE NO.37 CACAO
zinc oxide, octinoxate, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-756 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MANGANESE SULFATE (UNII: W00LYS4T26) ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-756-31 2 in 1 CARTON 12/26/2016 04/09/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/26/2016 04/09/2021 Labeler - Amorepacific Corporation (631035289) Establishment Name Address ID/FEI Business Operations Amorepacific Corporation 694894112 MANUFACTURE(43419-751, 43419-752, 43419-753, 43419-754, 43419-755, 43419-756)