| NDC | 49967-222-01 |
| Set ID | 25f68a1a-6d39-4019-85fa-4a0d8c656c19 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | L'Oreal USA Products Inc |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
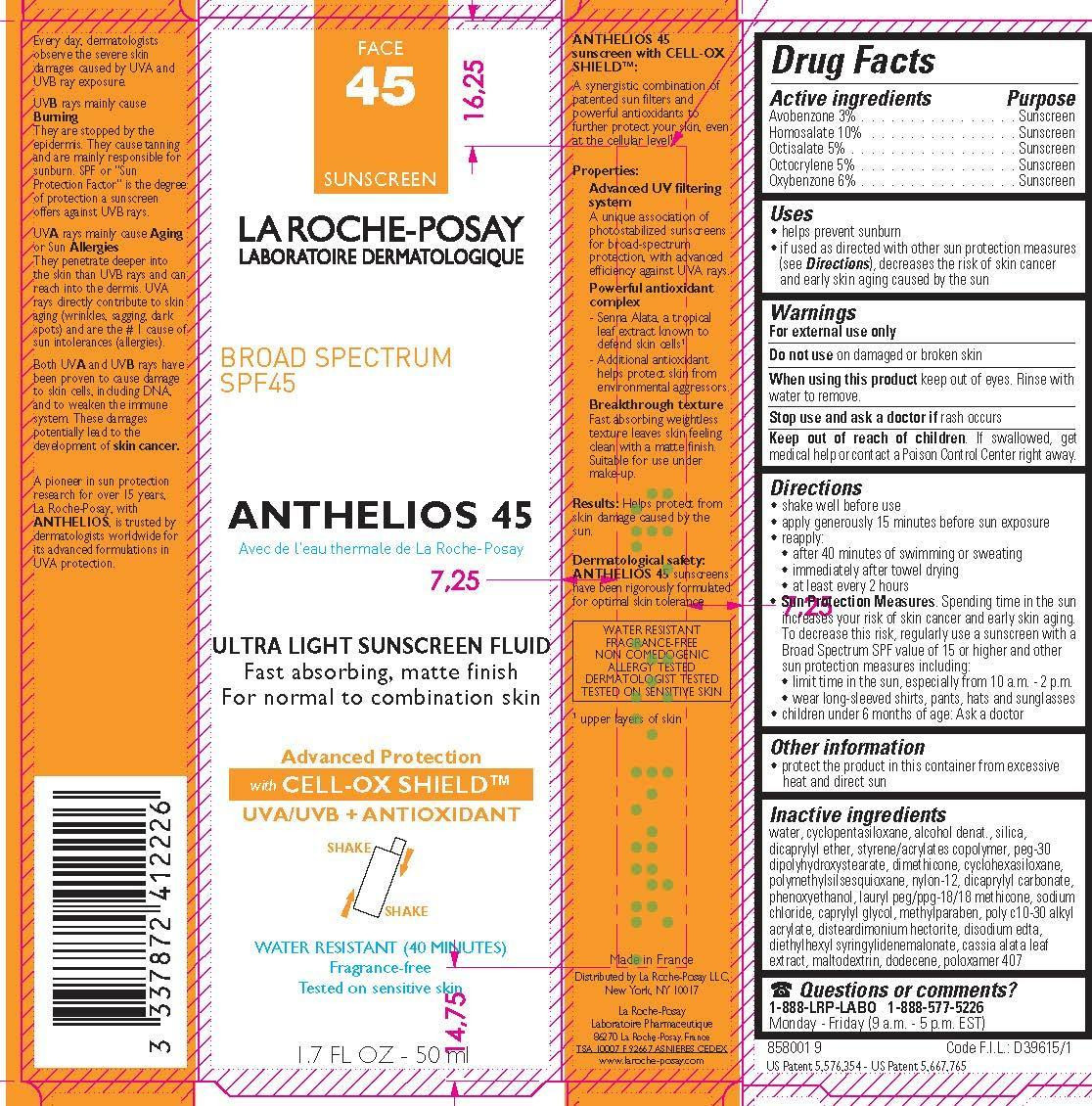
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
● apply generously 15 minutes before sun exposure
● reapply:
● after 40 minutes of swimming or sweating
● immediately after towel drying
● at least every 2 hours
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, cyclopentasiloxane, alcohol denat., silica, dicaprylyl ether, styrene/acrylates copolymer, PEG-30 dipolyhydroxystearate, dimethicone, cyclohexsiloxane, polymethylsilsesquioxane, nylon-12, dicaprylyl carbonate, phenoxyethanol, lauryl PEG/PPG-18/18 methicone, sodium chloride, caprylyl glycol, methylparaben, poly c10-30 alkyl acrylate, disteardimonium hectorite, disodium EDTA, diethylhexyl syringylidenemalonate, cassia alata leaf extract, maltodextrin, dodecene, poloxamer 407
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 45 ULTRA LIGHT SUNSCREEN FLUID WATER RESISTANT 40
avobenzone, homosalate, octisalate, octocrylene and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 50 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DICAPRYLYL ETHER (UNII: 77JZM5516Z) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 6 (UNII: XHK3U310BA) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) NYLON-12 (UNII: 446U8J075B) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) PHENOXYETHANOL (UNII: HIE492ZZ3T) LAURYL PEG/PPG-18/18 METHICONE (UNII: ZJ5S27D9NX) SODIUM CHLORIDE (UNII: 451W47IQ8X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) METHYLPARABEN (UNII: A2I8C7HI9T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) MALTODEXTRIN (UNII: 7CVR7L4A2D) DODECANE (UNII: 11A386X1QH) POLOXAMER 407 (UNII: TUF2IVW3M2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-222-01 1 in 1 CARTON 04/01/2010 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/01/2010 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Cosmetique Active Production 282658798 manufacture(49967-222) , analysis(49967-222) Establishment Name Address ID/FEI Business Operations BCM COSMETIQUE SAS 275359578 pack(49967-222)