| NDC | 25047-761-44, 25047-762-44, 25047-763-44, 25047-764-44 |
| Set ID | 427f25e6-e50a-407a-a6f0-512123e381e4 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | DMG AMERICA, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- INDICATIONS
- CONTAINS
- WARNING
- EMERGENCY RESPONSE
-
INSTRUCTIONS FOR USE
kolorz®
By DMG America
Instructions For UseKolorz Sixty Second Fluoride Foam
Kolorz Neutral Fluoride Foam
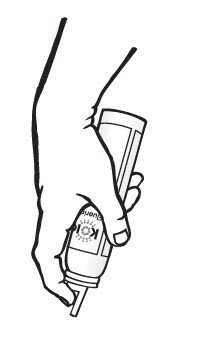
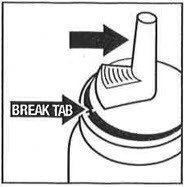
The first time you dispense from a new bottle, gently lift upward on the nozzle to break the protective shipping tab (thin plastic tab located adjacent to "trigger"). If this tab is not broken, there could be an initial surge of foam from the dispenser.

Dosage and Administration:
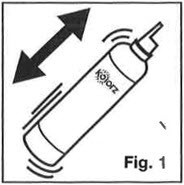
- Shake can vigorously before each use. (Fig. 1)

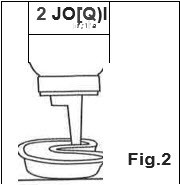
- Turn can completely upside down to dispense. Point can towards fluoride applicator tray and slowly press the nozzle to fill tray. Use one press per arch, as foam will expand slightly to fill the tray. (Fig. 2)

- Dry tooth surface and insert tray(s) in mouth.
- For Kolorz Sixty Second Fluoride Foam: Have patient bite down lightly but firmly for 1 to 4 minutes; For Kolorz Neutral Fluoride Foam: Have patient bite down lightly but firmly for 4 minutes.
- Remove tray(s) and have patient expectorate excess
- Instruct patient not to eat, drink, or rinse for at least 30 minutes after application.
How Supplied: Both Kolorz Sixty Second Fluoride Foam and Kolorz Neutral Fluoride Foam are available in 4.4oz. (125g) cans and do not contain chlorofluorocarbon propellant.
Caution: For professional use only. Federal U.S.A. Law prohibits dispensing without prescription.
Warning: Contents under pressure. Do not puncture or incinerate. Store between 55-80° F. Keep from freezing.
kolorz®
Distributed by DMG America
242 South Dean Street
Englewood, NJ 07631 USA
Tel: 201-894-5505
Fax: 201-894-0213
www.dmg-america.com395490 Rev. 11/13
- Shake can vigorously before each use. (Fig. 1)
- PRINCIPAL DISPLAY PANEL - 125 g Can Carton - Blue Raspberry
- PRINCIPAL DISPLAY PANEL - 125 g Can Carton - Cherry Cheesecake
- PRINCIPAL DISPLAY PANEL - 125 g Can Carton - Cotton Candy
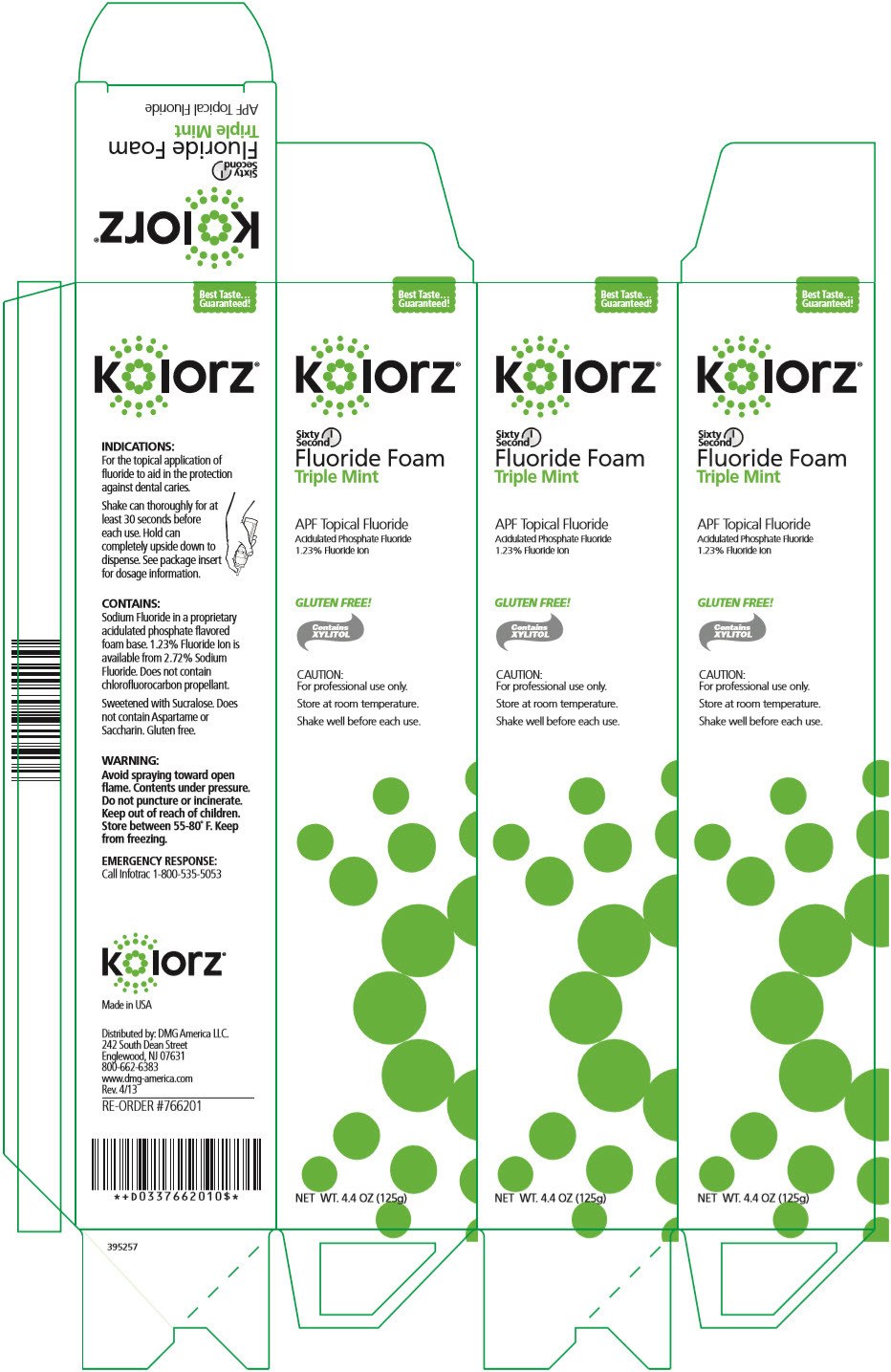
- PRINCIPAL DISPLAY PANEL - 125 g Can Carton - Triple Mint
-
INGREDIENTS AND APPEARANCE
KOLORZ SIXTY SECOND FLUORIDE FOAM BLUE RASPBERRY
sodium fluoride aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-764 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1.23 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor RASPBERRY (BLUE RASPBERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-764-44 125 g in 1 CAN; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE FOAM CHERRY CHEESECAKE
sodium fluoride aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-762 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1.23 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor CHERRY (CHERRY CHEESECAKE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-762-44 125 g in 1 CAN; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE FOAM COTTON CANDY
sodium fluoride aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-763 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1.23 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor COTTON CANDY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-763-44 125 g in 1 CAN; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE FOAM TRIPLE MINT
sodium fluoride aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-761 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1.23 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor MINT (TRIPLE MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-761-44 125 g in 1 CAN; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 Labeler - DMG AMERICA, LLC (106792427)