| NDC | 25047-771-16, 25047-772-16, 25047-773-16, 25047-774-16, 25047-775-16 |
| Set ID | 21fa26f2-0162-4044-aec0-d5360be3f3e0 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | DMG AMERICA, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- INDICATIONS
-
DOSAGE & ADMINISTRATION
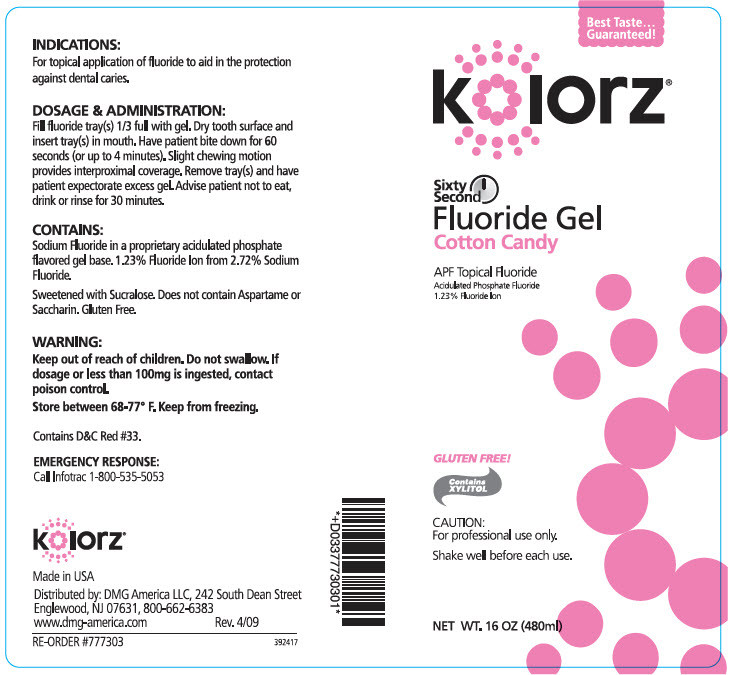
Fill fluoride tray(s) 1/3 full with gel. Dry tooth surface and insert tray(s) in mouth. Have patient bite down for 60 seconds (or up to 4 minutes). Slight chewing motion provides interproximal coverage. Remove tray(s) and have patient expectorate excess gel. Advise patient not to eat, drink or rinse for 30 minutes.
- CONTAINS
- WARNING
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- EMERGENCY RESPONSE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cherry Cheesecake
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Piña Colada
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Triple Mint
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Blue Raspberry
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cotton Candy
-
INGREDIENTS AND APPEARANCE
KOLORZ SIXTY SECOND FLUORIDE CHERRY CHEESECAKE
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-772 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor CHERRY (CHERRY CHEESECAKE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-772-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE PINA COLADA
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-775 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor PINEAPPLE (PIÑA COLADA) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-775-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE TRIPLE MINT
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-771 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor MINT (TRIPLE MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-771-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE BLUE RASPBERRY
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-774 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor RASPBERRY (BLUE RASPBERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-774-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE COTTON CANDY
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-773 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor COTTON CANDY (COTTON CANDY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-773-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 Labeler - DMG AMERICA, LLC (106792427)