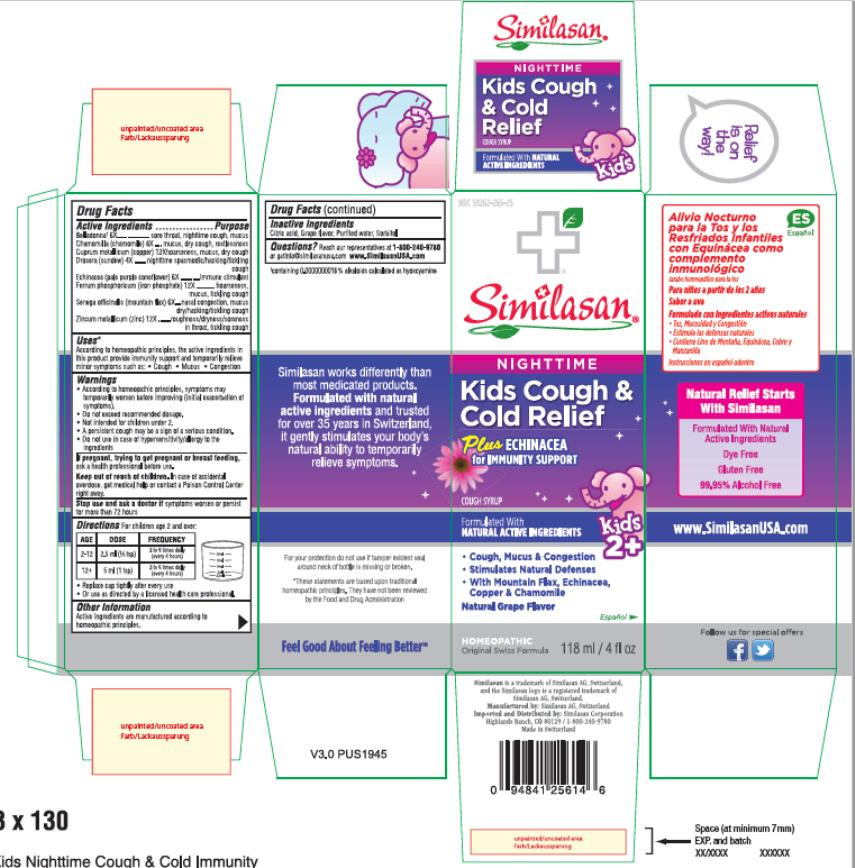
| NDC | 59262-265-25 |
| Set ID | 58e27b17-31bf-4950-8bc0-88c725109c35 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Similasan Corporation |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses*
-
Warnings
- According to homeopathic principles, symptoms may temporarily worsen before improving (initial exacerbation of symptoms).
- Do not exceed recommended dosage.
- Not intended for children under 2.
- A persistent cough may be a sign of a serious condition.
- Do not use in case of hypersensitivity/allergy to the ingredients
- According to homeopathic principles, symptoms may temporarily worsen before improving (initial exacerbation of symptoms).
- Directions
- Other Information:
- Inactive Ingredients:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KIDS COUGH AND COLD RELIEF PLUS ECHINACEA, NIGHTTIME
atropa belladonna, matricaria recutita, copper, drosera rotundifolia flowering top, echinacea pallida, ferrosoferric phosphate, polygala senega root and zinc syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-265 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] in 1 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 8 [hp_X] in 1 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 1 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 4 [hp_X] in 1 mL ECHINACEA PALLIDA (UNII: 904CK3270L) (ECHINACEA PALLIDA - UNII:904CK3270L) ECHINACEA PALLIDA 6 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 1 mL POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 6 [hp_X] in 1 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-265-25 1 in 1 BOX 02/01/2016 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 02/01/2016 Labeler - Similasan Corporation (111566530)