| NDC | 71742-0011-1 |
| Set ID | c1845e0f-8171-4278-a838-324d1cdfbd4b |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guangzhou Renuma Medical Systems Co., Ltd |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
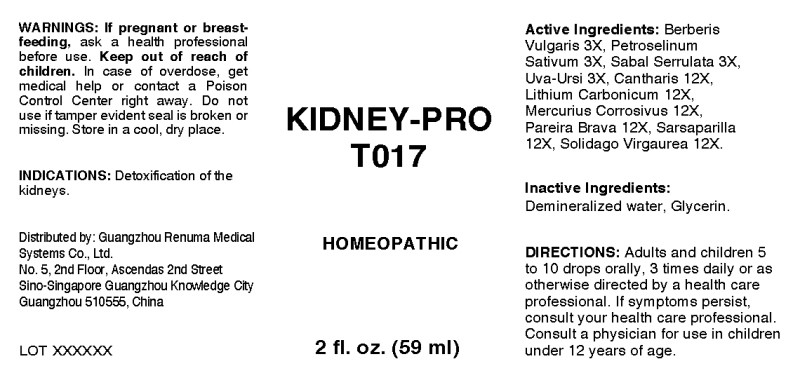
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
KIDNEY-PRO T017
berberis vulgaris, petroselinum sativum, sabal serrulata, uva ursi, cantharis, lithium carbonicum, mercurius corrosivus, pareira brava, sarsaparilla (smilax regelii), solidago virgaurea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71742-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 1 mL PETROSELINUM CRISPUM (UNII: 1WZA4Y92EX) (PETROSELINUM CRISPUM - UNII:1WZA4Y92EX) PETROSELINUM CRISPUM 3 [hp_X] in 1 mL SAW PALMETTO (UNII: J7WWH9M8QS) (SAW PALMETTO - UNII:J7WWH9M8QS) SAW PALMETTO 3 [hp_X] in 1 mL ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) (ARCTOSTAPHYLOS UVA-URSI LEAF - UNII:3M5V3D1X36) ARCTOSTAPHYLOS UVA-URSI LEAF 3 [hp_X] in 1 mL LYTTA VESICATORIA (UNII: 3Q034RO3BT) (LYTTA VESICATORIA - UNII:3Q034RO3BT) LYTTA VESICATORIA 12 [hp_X] in 1 mL LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 12 [hp_X] in 1 mL MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 12 [hp_X] in 1 mL CHONDRODENDRON TOMENTOSUM ROOT (UNII: 395A3P448Z) (CHONDRODENDRON TOMENTOSUM ROOT - UNII:395A3P448Z) CHONDRODENDRON TOMENTOSUM ROOT 12 [hp_X] in 1 mL SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SMILAX ORNATA ROOT - UNII:2H1576D5WG) SMILAX ORNATA ROOT 12 [hp_X] in 1 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71742-0011-1 59 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/26/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/26/2017 Labeler - Guangzhou Renuma Medical Systems Co., Ltd (544538706) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(71742-0011) , api manufacture(71742-0011) , label(71742-0011) , pack(71742-0011)