| NDC | 76049-121-01, 76049-122-01, 76049-123-01 |
| Set ID | 37154826-c34a-416d-a9a3-5004156dccd6 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Stila Styles |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
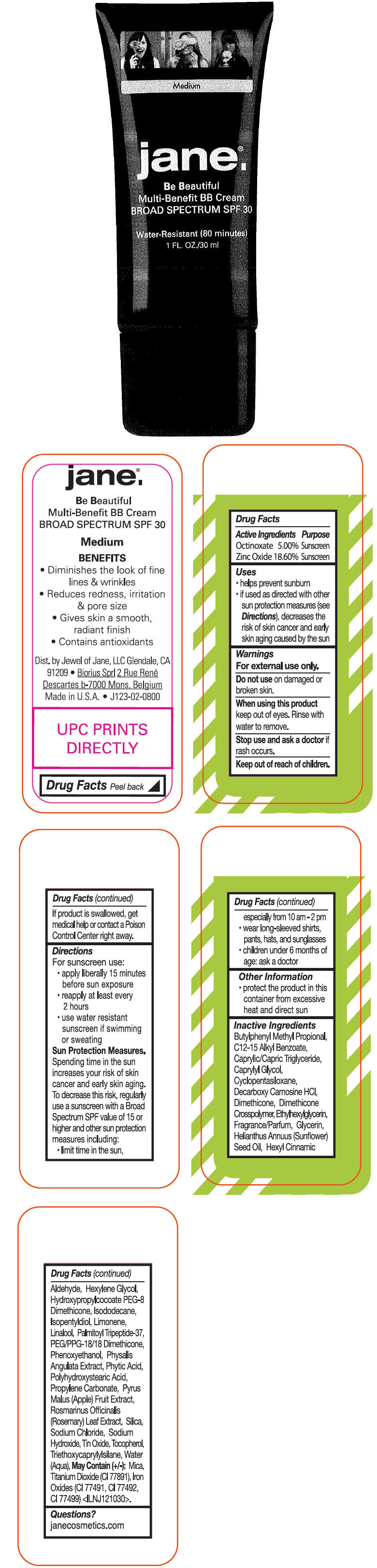
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use water resistant sunscreen if swimming or sweating
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 am - 2 pm
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: ask a doctor
- Other Information
-
Inactive Ingredients
Butylphenyl Methyl Propional, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cyclopentasiloxane, Decarboxy Carnosine HCl, Dimethicone, Dimethicone Crosspolymer, Ethylhexylglycerin, Fragrance/Parfum, Glycerin, Helianthus Annuus (Sunflower) Seed Oil, Hexyl Cinnamic Aldehyde, Hexylene Glycol, Hydroxypropylcocoate PEG-8 Dimethicone, Isododecane, Isopentyldiol, Limonene, Linalool, Palmitoyl Tripeptide-37, PEG/PPG-18/18 Dimethicone, Phenoxyethanol, Physalis Angulata Extract, Phytic Acid, Polyhydroxystearic Acid, Propylene Carbonate, Pyrus Malus (Apple) Fruit Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Silica, Sodium Chloride, Sodium Hydroxide, Tin Oxide, Tocopherol, Triethoxycaprylylsilane, Water (Aqua), May Contain (+/-): Mica, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499) <ILNJ121030>.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Tube - Light
- PRINCIPAL DISPLAY PANEL - 30 ml Tube - Medium
- PRINCIPAL DISPLAY PANEL - 30 ml Tube - Dark
-
INGREDIENTS AND APPEARANCE
JANE BE BEAUTIFUL MULTI-BENEFIT BB BROAD SPECTRUM SPF 30 LIGHT
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76049-123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 186 mg in 1 mL Inactive Ingredients Ingredient Name Strength Butylphenyl Methylpropional (UNII: T7540GJV69) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Caprylyl Glycol (UNII: 00YIU5438U) Cyclomethicone 5 (UNII: 0THT5PCI0R) Decarboxy Carnosine Hydrochloride (UNII: 6X7K9I5QR7) Dimethicone (UNII: 92RU3N3Y1O) Ethylhexylglycerin (UNII: 147D247K3P) Glycerin (UNII: PDC6A3C0OX) Sunflower Oil (UNII: 3W1JG795YI) .Alpha.-Hexylcinnamaldehyde (UNII: 7X6O37OK2I) Hexylene Glycol (UNII: KEH0A3F75J) Hydroxypropylcocoate Peg-8 Dimethicone (UNII: 8TE0BZU36S) Isododecane (UNII: A8289P68Y2) Isopentyldiol (UNII: 19NOL5474Q) Linalool, (+/-)- (UNII: D81QY6I88E) PEG/PPG-18/18 Dimethicone (UNII: 9H0AO7T794) Phenoxyethanol (UNII: HIE492ZZ3T) Physalis Angulata (UNII: W4TKW9D5GG) Fytic Acid (UNII: 7IGF0S7R8I) Propylene Carbonate (UNII: 8D08K3S51E) Apple (UNII: B423VGH5S9) Rosemary (UNII: IJ67X351P9) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Tocopherol (UNII: R0ZB2556P8) Triethoxycaprylylsilane (UNII: LDC331P08E) Water (UNII: 059QF0KO0R) Mica (UNII: V8A1AW0880) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Red (UNII: 1K09F3G675) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferrosoferric Oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76049-123-01 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 10/31/2013 JANE BE BEAUTIFUL MULTI-BENEFIT BB BROAD SPECTRUM SPF 30 MEDIUM
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76049-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 186 mg in 1 mL Inactive Ingredients Ingredient Name Strength Butylphenyl Methylpropional (UNII: T7540GJV69) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Caprylyl Glycol (UNII: 00YIU5438U) Cyclomethicone 5 (UNII: 0THT5PCI0R) Decarboxy Carnosine Hydrochloride (UNII: 6X7K9I5QR7) Dimethicone (UNII: 92RU3N3Y1O) Ethylhexylglycerin (UNII: 147D247K3P) Glycerin (UNII: PDC6A3C0OX) Sunflower Oil (UNII: 3W1JG795YI) .Alpha.-Hexylcinnamaldehyde (UNII: 7X6O37OK2I) Hexylene Glycol (UNII: KEH0A3F75J) Hydroxypropylcocoate Peg-8 Dimethicone (UNII: 8TE0BZU36S) Isododecane (UNII: A8289P68Y2) Isopentyldiol (UNII: 19NOL5474Q) Linalool, (+/-)- (UNII: D81QY6I88E) PEG/PPG-18/18 Dimethicone (UNII: 9H0AO7T794) Phenoxyethanol (UNII: HIE492ZZ3T) Physalis Angulata (UNII: W4TKW9D5GG) Fytic Acid (UNII: 7IGF0S7R8I) Propylene Carbonate (UNII: 8D08K3S51E) Apple (UNII: B423VGH5S9) Rosemary (UNII: IJ67X351P9) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Stannic Oxide (UNII: KM7N50LOS6) Tocopherol (UNII: R0ZB2556P8) Triethoxycaprylylsilane (UNII: LDC331P08E) Water (UNII: 059QF0KO0R) Mica (UNII: V8A1AW0880) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Red (UNII: 1K09F3G675) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferrosoferric Oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76049-122-01 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 10/31/2013 JANE BE BEAUTIFUL MULTI-BENEFIT BB BROAD SPECTRUM SPF 30 DARK
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76049-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 186 mg in 1 mL Inactive Ingredients Ingredient Name Strength Butylphenyl Methylpropional (UNII: T7540GJV69) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Caprylyl Glycol (UNII: 00YIU5438U) Cyclomethicone 5 (UNII: 0THT5PCI0R) Decarboxy Carnosine Hydrochloride (UNII: 6X7K9I5QR7) Dimethicone (UNII: 92RU3N3Y1O) Ethylhexylglycerin (UNII: 147D247K3P) Glycerin (UNII: PDC6A3C0OX) Sunflower Oil (UNII: 3W1JG795YI) .Alpha.-Hexylcinnamaldehyde (UNII: 7X6O37OK2I) Hexylene Glycol (UNII: KEH0A3F75J) Hydroxypropylcocoate Peg-8 Dimethicone (UNII: 8TE0BZU36S) Isododecane (UNII: A8289P68Y2) Isopentyldiol (UNII: 19NOL5474Q) Linalool, (+/-)- (UNII: D81QY6I88E) PEG/PPG-18/18 Dimethicone (UNII: 9H0AO7T794) Phenoxyethanol (UNII: HIE492ZZ3T) Physalis Angulata (UNII: W4TKW9D5GG) Fytic Acid (UNII: 7IGF0S7R8I) Propylene Carbonate (UNII: 8D08K3S51E) Apple (UNII: B423VGH5S9) Rosemary (UNII: IJ67X351P9) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Tocopherol (UNII: R0ZB2556P8) Triethoxycaprylylsilane (UNII: LDC331P08E) Water (UNII: 059QF0KO0R) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Red (UNII: 1K09F3G675) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferrosoferric Oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76049-121-01 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 10/31/2013 Labeler - Stila Styles (809192896) Establishment Name Address ID/FEI Business Operations GORDON LABORATORIES, INC. 008328619 MANUFACTURE(76049-123, 76049-122, 76049-121)