| NDC | 72501-071-18, 72501-071-19, 72501-072-01, 72501-072-02, 72501-073-94, 72501-073-95, 72501-074-48, 72501-074-49, 72501-075-70, 72501-075-71, 72501-076-87, 72501-077-25, 72501-077-26, 72501-078-32 |
| Set ID | 71a557b7-d7b0-7622-e053-2a95a90aeb60 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Shanghai Iceking Biotechnology Co.,Ltd. |
| Generic Name | |
| Product Class | Anti-coagulant |
| Product Number | |
| Application Number |
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening freckle removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening freckle removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening freckle removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening freckle removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
- iceking heparin scar repair gel
- iceking whitening freckle removing essence lotion
- iceking heparin scar repair gel
- iceking anti-onychomycosis gel
- iceking freckle removing whitening masque
- iceking repellent mosquito spray
- iceking whitening freckle removing essence lotion
- iceking freckle removing whitening facial cleanser
- iceking refresh armpit spray
- iceking refreshing body roller
-
INGREDIENTS AND APPEARANCE
ICEKING FRECKLE REMOVING WHITENING FACIAL CLEANSER
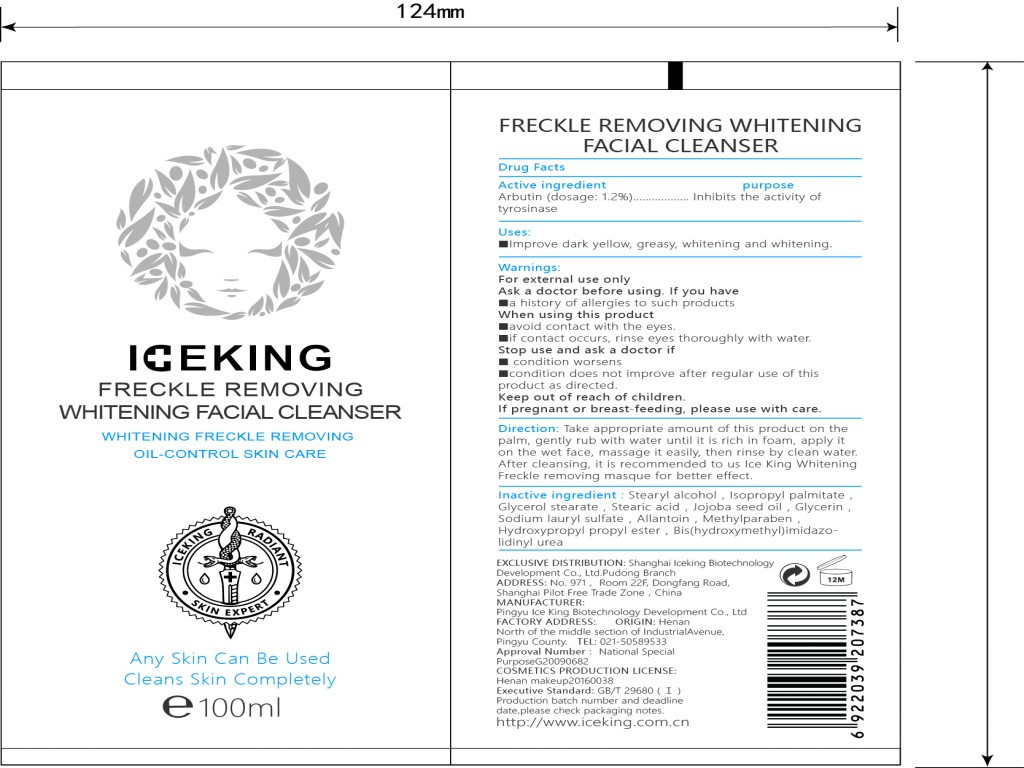
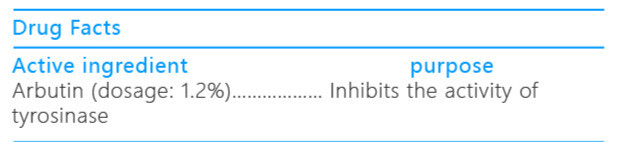
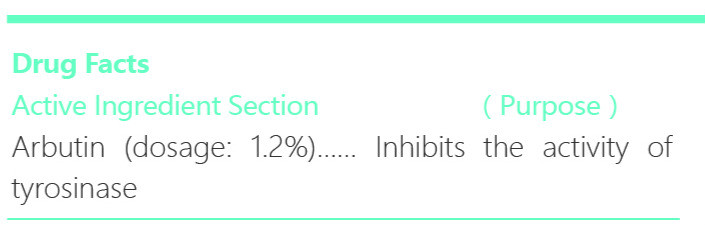
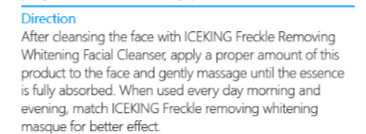
iceking freckle removing whitening facial cleanser ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-076 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARBUTIN (UNII: C5INA23HXF) (ARBUTIN - UNII:C5INA23HXF) ARBUTIN 1.2 g in 5 mL Inactive Ingredients Ingredient Name Strength STEARYL ALCOHOL (UNII: 2KR89I4H1Y) STEARIC ACID (UNII: 4ELV7Z65AP) JOJOBA OIL (UNII: 724GKU717M) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM LAURYL SULFATE (UNII: 368GB5141J) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) Product Characteristics Color white Score Shape Size Flavor VANILLA (potpourri) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-076-87 1 mL in 1 TUBE; Type 0: Not a Combination Product 09/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING FRECKLE REMOVING WHITENING MASQUE
iceking freckle removing whitening masque liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-073 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARBUTIN (UNII: C5INA23HXF) (ARBUTIN - UNII:C5INA23HXF) ARBUTIN 0.36 g in 5 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HYALURONIC ACID (UNII: S270N0TRQY) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-8 DIMETHICONE (UNII: GIA7T764OD) Product Characteristics Color Score Shape Size Flavor VANILLA (potpourri) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-073-95 1 in 1 BOX 09/15/2018 1 NDC:72501-073-94 1 g in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING REPELLENT MOSQUITO
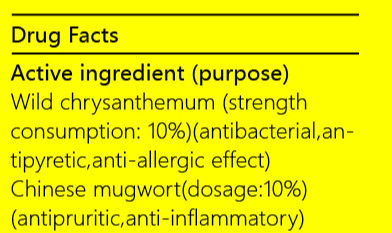
iceking repellent mosquito sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-074 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHRYSANTHEMUM INDICUM FLOWER (UNII: I6OER6U04L) (CHRYSANTHEMUM INDICUM FLOWER - UNII:I6OER6U04L) CHRYSANTHEMUM INDICUM FLOWER 6 g in 5 mL MENTHA ARVENSIS LEAF (UNII: A4IWO4DDZ9) (MENTHA ARVENSIS LEAF - UNII:A4IWO4DDZ9) MENTHA ARVENSIS LEAF 6 g in 5 mL ARTEMISIA ARGYI LEAF (UNII: 2JYC99Q0WZ) (ARTEMISIA ARGYI LEAF - UNII:2JYC99Q0WZ) ARTEMISIA ARGYI LEAF 6 g in 5 mL Inactive Ingredients Ingredient Name Strength THYMOL (UNII: 3J50XA376E) BORNEOL (UNII: M89NIB437X) ALCOHOL (UNII: 3K9958V90M) MENTHYL D-LACTATE, (-)- (UNII: XFS8QYW6WY) Product Characteristics Color yellow (faint yellow) Score Shape Size Flavor LICORICE (Plant smell) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-074-49 1 in 1 BOX, UNIT-DOSE 09/15/2018 1 NDC:72501-074-48 1 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING SKIN EXPERT HEPARIN SCAR REPAIR GEL
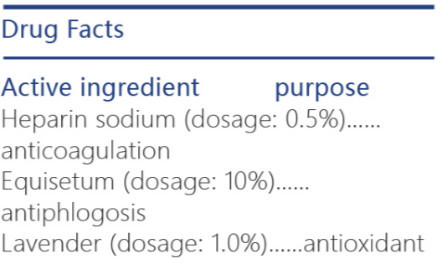
iceking skin expert heparin scar repair gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-071 Route of Administration INFILTRATION, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 2 g in 5 g LAVENDER OIL (UNII: ZBP1YXW0H8) (LAVENDER OIL - UNII:ZBP1YXW0H8) LAVENDER OIL 0.02 g in 5 g EQUISETUM HYEMALE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE 2 g in 5 g HEPARIN SODIUM (UNII: ZZ45AB24CA) (HEPARIN - UNII:T2410KM04A) HEPARIN 0.1 g in 5 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYOXYL 60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROLINE (UNII: 9DLQ4CIU6V) SODIUM PIDOLATE (UNII: 1V74VH163T) Product Characteristics Color brown Score Shape Size Flavor VANILLA (lavendar smell) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-071-19 1 in 1 BOX, UNIT-DOSE 09/15/2018 1 NDC:72501-071-18 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING REFRESH ARMPIT
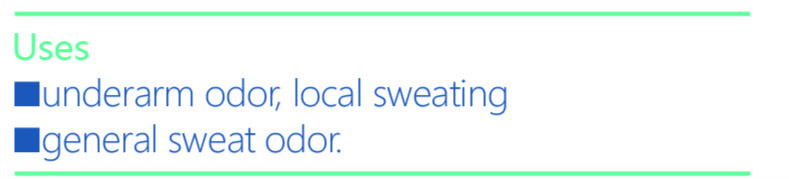
iceking refresh armpit sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-077 Route of Administration INFILTRATION, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE ANHYDROUS (UNII: 407PSC3OC7) (ALUMINUM CHLOROHYDRATE ANHYDROUS - UNII:407PSC3OC7) ALUMINUM CHLOROHYDRATE ANHYDROUS 9 g in 5 g Inactive Ingredients Ingredient Name Strength POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) IMIDUREA (UNII: M629807ATL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white (lucid) Score Shape Size Flavor VANILLA (potpourri) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-077-26 1 in 1 BOX, UNIT-DOSE 09/15/2018 1 NDC:72501-077-25 1 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING WHITENING FRECKLE REMOVING ESSENCE
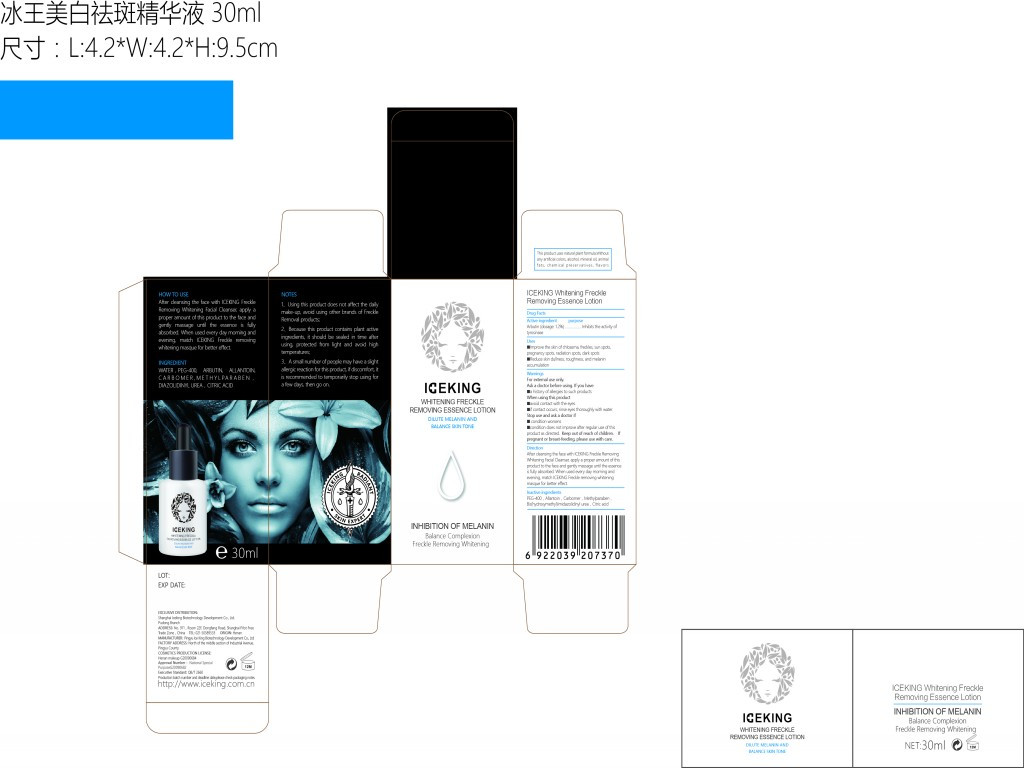
iceking whitening freckle removing essence lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-075 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARBUTIN (UNII: C5INA23HXF) (ARBUTIN - UNII:C5INA23HXF) ARBUTIN 0.36 g in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) METHYLPARABEN (UNII: A2I8C7HI9T) ALLANTOIN (UNII: 344S277G0Z) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-075-71 1 in 1 BOX, UNIT-DOSE 09/15/2018 1 NDC:72501-075-70 1 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING SKIN EXPERT ANTI-ONYCHOMYCOSIS GEL
iceking skin expert anti-onychomycosis gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-072 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BORIC ACID (UNII: R57ZHV85D4) (BORIC ACID - UNII:R57ZHV85D4) BORIC ACID 0.2 g in 5 g ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 2 g in 5 g Inactive Ingredients Ingredient Name Strength PHELLODENDRON AMURENSE BARK (UNII: PBG27B754G) PSEUDOLARIX AMABILIS WHOLE (UNII: G2TMJ38TAU) Product Characteristics Color brown (rufous) Score Shape Size Flavor FRUIT (acetic acid) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-072-02 1 in 1 BOX, UNIT-DOSE 09/15/2018 1 NDC:72501-072-01 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 ICEKING SKIN EXPERT REFRESHING BODY ROLLER
iceking skin expert refreshing body roller liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72501-078 Route of Administration CUTANEOUS, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE (UNII: R4KO0DY52L) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE 0.0375 g in 5 mL BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.075 g in 5 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) BORIC ACID (UNII: R57ZHV85D4) ALCOHOL (UNII: 3K9958V90M) Product Characteristics Color white Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72501-078-32 1 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product 09/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/15/2018 Labeler - Shanghai Iceking Biotechnology Co.,Ltd. (526903007) Establishment Name Address ID/FEI Business Operations Shanghai Iceking Biotechnology Co.,Ltd. 526903007 manufacture(72501-071, 72501-072, 72501-073, 72501-074, 72501-075, 72501-076, 72501-077, 72501-078)