| NDC | 57826-462-03 |
| Set ID | e6775fa2-9f23-4d71-be07-1e9e4d6265d0 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Haemonetics Corporation |
| Generic Name | |
| Product Class | Anti-coagulant |
| Product Number | |
| Application Number | BLA000127 |
-
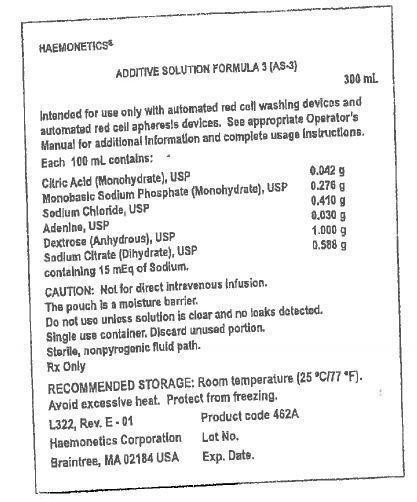
Additive Solution Formula 3 (AS-3) 300 mL
Intended for use only with automated red cell apheresis devices. See Operator's Manual for additional Information and complete usage instructions.
Each 100 mL contains:
Citric Acid (Monohydrate), USP 0.042 g
Monobasic Sodium Phosphate (Monohydrate), USP 0.276 g
Sodium Chloride, USP 0.410 g
Adenine, USP 0.030 g
Dextrose (Anhydrous), USP 1.000 g
Sodium Citrate (Dihydrate), USP 0.588 g
containing 15 mEq of Sodium. - CAUTION:
- STERILE,
- RECOMMENDED STORAGE:
- CAUTION:
- PRINCIPAL DISPLAY PANEL
- Product Label - Package
-
INGREDIENTS AND APPEARANCE
HAEMONETICS ADDITIVE SOLUTION FORMULA 3 (AS-3)
citric acid monohydrate, sodium phosphate, monobasic, monohydrate, sodium chloride, adenine, anhydrous dextrose, trisodium citrate dihydrate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57826-462 Route of Administration EXTRACORPOREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 4.2 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 27.6 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698, SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 41 mg in 1 mL ADENINE (UNII: JAC85A2161) (ADENINE - UNII:JAC85A2161) ADENINE 3 mg in 1 mL ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) ANHYDROUS DEXTROSE 100 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 58.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57826-462-03 300 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN000127 01/07/2013 Labeler - Haemonetics Corporation (057827420) Establishment Name Address ID/FEI Business Operations Haemonetics Manufacturing Inc. 078598396 manufacture(57826-462) Establishment Name Address ID/FEI Business Operations Haemonetics Corporation 942344649 manufacture(57826-462)