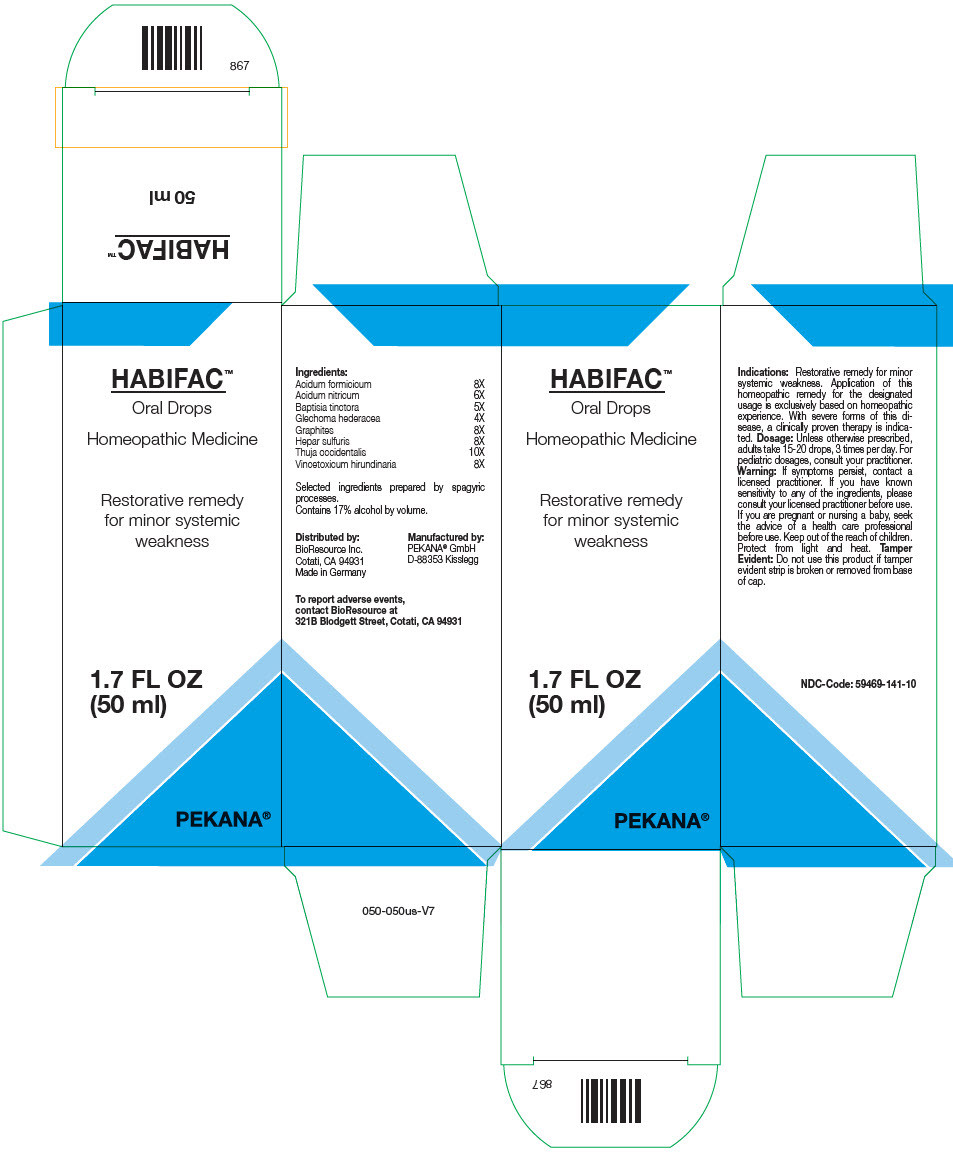
| NDC | 59469-141-10 |
| Set ID | b5fd91ae-3822-421a-a449-4178111950bc |
| Category | HUMAN OTC DRUG LABEL |
| Packager | PEKANA Naturheilmittel GmbH |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- Indications
- Dosage
- Warning
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
HABIFAC
formic acid, nitric acid, baptisia tinctoria root, graphite, calcium sulfide, thuja occidentalis leafy twig, cynanchum vincetoxicum leaf, and glechoma hederacea flowering top solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59469-141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Formic Acid (UNII: 0YIW783RG1) (Formic Acid - UNII:0YIW783RG1) Formic Acid 8 [hp_X] in 50 mL Nitric Acid (UNII: 411VRN1TV4) (Nitric Acid - UNII:411VRN1TV4) Nitric Acid 6 [hp_X] in 50 mL Baptisia tinctoria Root (UNII: 5EF0HWI5WU) (Baptisia Tinctoria Root - UNII:5EF0HWI5WU) Baptisia tinctoria Root 5 [hp_X] in 50 mL Graphite (UNII: 4QQN74LH4O) (Graphite - UNII:4QQN74LH4O) Graphite 8 [hp_X] in 50 mL Calcium sulfide (UNII: 1MBW07J51Q) (Calcium sulfide - UNII:1MBW07J51Q) Calcium sulfide 8 [hp_X] in 50 mL Thuja occidentalis Leafy Twig (UNII: 1NT28V9397) (Thuja occidentalis Leafy Twig - UNII:1NT28V9397) Thuja occidentalis Leafy Twig 10 [hp_X] in 50 mL Vincetoxicum Hirundinaria Leaf (UNII: 9WLP0FA6U4) (Vincetoxicum Hirundinaria Leaf - UNII:9WLP0FA6U4) Vincetoxicum Hirundinaria Leaf 8 [hp_X] in 50 mL Glechoma hederacea Flowering Top (UNII: 2458J91U39) (Glechoma Hederacea Flowering Top - UNII:2458J91U39) Glechoma hederacea Flowering Top 4 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59469-141-10 1 in 1 BOX 04/12/2008 1 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 04/12/2008 Labeler - PEKANA Naturheilmittel GmbH (320344542) Establishment Name Address ID/FEI Business Operations PEKANA Naturheilmittel GmbH 320344542 MANUFACTURE(59469-141)